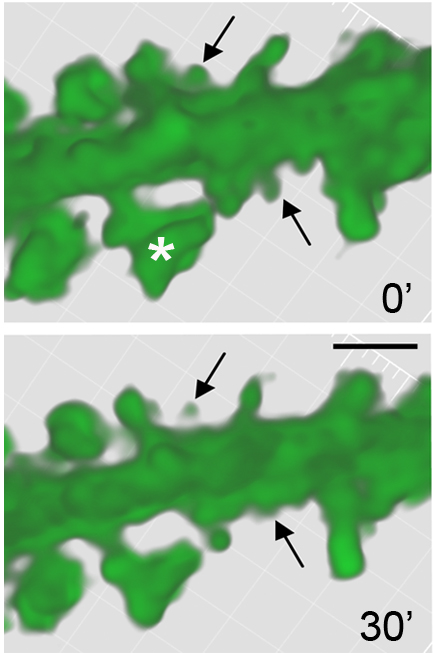
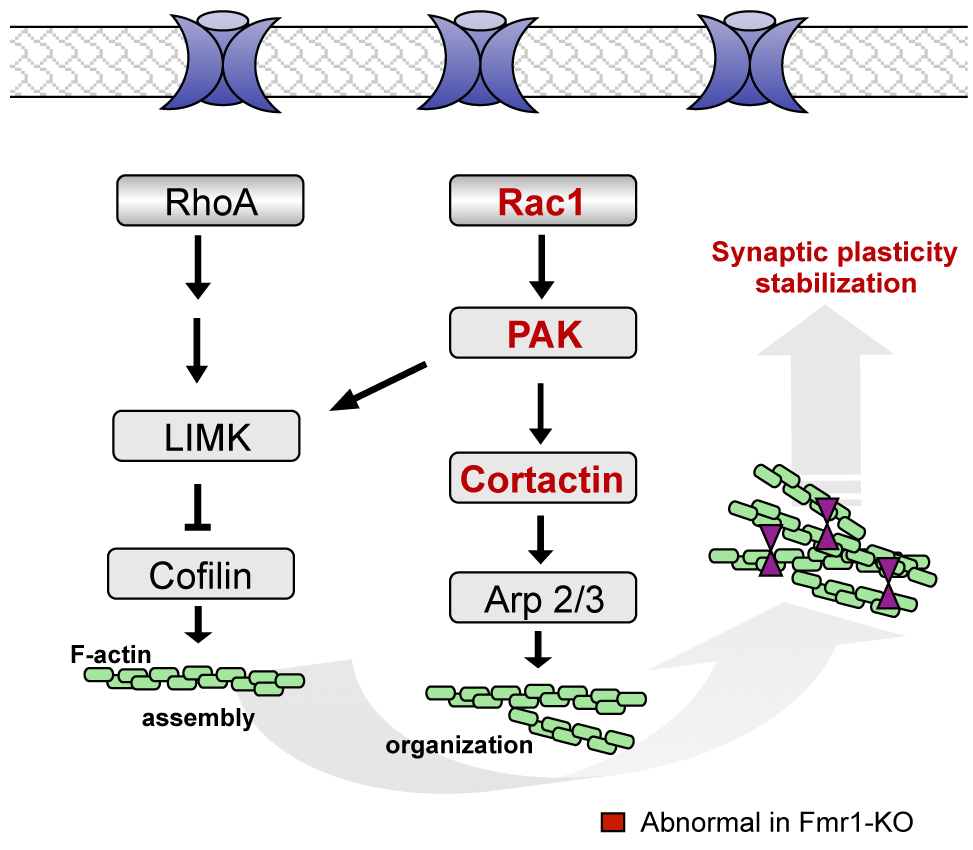
Fragile X (FX) syndrome and Down Syndrome ((DS) are developmental intellectual disability disorders, and both are characterized by abnormalities in the dendritic spines of cortical pyramidal cells. Dendritic spines are small protrusions on a neuron’s dendrite that receive input from other cells and are the site for synaptic strength and plasticity. Our work utilizes mouse models of both FX (Fmr1 knockout) and DS (Ts65Dn) to investigate how synaptic mechanisms that promote learning and memory are perturbed in these disorders. In particular, we have shown that Fmr1 knockout mice have deficient hippocampal Long Term Potentiation (LTP), the presumable cellular substrate of memory, and learning deficits. Moreover, we have found that signaling pathways that regulate the actin cytoskeleton in hippocampal dendritic spines are selectively perturbed in Fmr1 knockouts. Current studies are extending this work to evaluate the same signaling pathways in the Ts65Dn model of Down syndrome and in human DS biopsy cortical tissue to determine if both FX and DS share common defects.
A second focus of our work is on stress and understanding how stress hormones (glucocorticoids) influence synaptic plasticity. We have shown that the glucocorticoid receptor is present in hippocampal dendritic spines and rapidly influences signaling pathways to the actin cytoskeleton. This suggests that stress can have direct effects on learning and memory mechanisms, and that therapies that counter these effects may be very beneficial. In addition, we have shown that Fmr1 KOs have a dysfunctional stress response similar to the human FX condition. Present studies are aimed at elucidating how this dysregulated stress response contributes to the FX behavioral phenotype, most particularly those relating to autism and intellect.
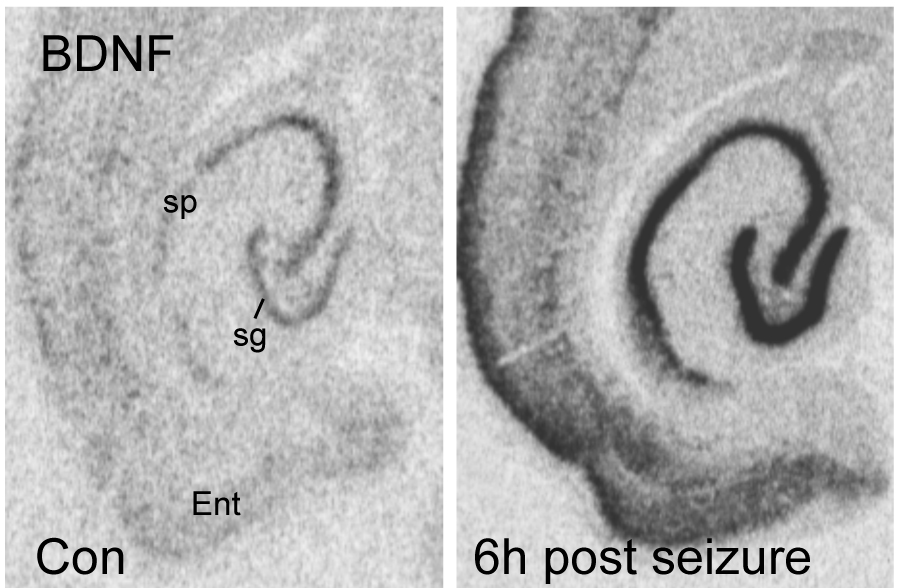
Finally, another major focus of our work is on developing therapeutic strategies for enhancing learning and memory and treating intellectual disabilities. We were the first to shown that positive AMPA receptor modulators, which increase AMPA receptor function, also increase levels of the neurotrophin brain derived neurotrophic factor (BDNF) in brain. Our studies also demonstrate that BDNF treatment restores hippocampal LTP in Fmr1 knockout mice, suggesting that interventions that target AMPA-class receptors and augment neurotrophin availability, such as ampakine treatment, could be of tremendous value for correcting abnormalities in dendritic spines and synaptic function associated with intellectual disability. We also have recently demonstrated that long-term ampakine treatment stimulates dendritic growth and promotes learning in middle-aged rats, thus forestalling the effects of aging on the brain.