Understanding the Metastatic Tumor Microenvironment in Pancreatic Cancer
Pancreatic is aggressively metastatic, most often to the liver, and usually found to be widely disseminated by the time of diagnosis. In the 20% of cases where localized disease is found sufficiently early, surgical resection is the most effective treatment option; however, ~80% of these patients succumb to recurrence of the disease. As a result, nearly all patients die with metastatic or recurrent disease. Accordingly, effective treatment of metastatic disease is urgently needed to improve outcomes of PDA.
Direct comparison of the pancreatic vs. liver tumor microenvironments has been complicated as most samples are not matched within the same patients leading to data clustering driven primarily by genetic diversity between patients instead of by tumor site. To overcome this limitation, we developed an experimental model directly contrasting orthotopic tumor injections in the mouse pancreas with a model of metastasis by injecting the same cancer cells into the liver through the splenic vein. Using this model, we have performed an unbiased single-cell RNA sequencing comparison of liver and pancreatic lesions, and a parallel 30-marker mass cytometry (CyTOF) immune profiling to measure differences in the cell populations in these tumors. Using these we have identified several unique cell populations and phenotypic behaviors unique to each lesion that we are working to target.
Characterizing and Targeting Metabolic Mechanisms of Chemoresistance in Pancreatic Cancer
One of the largest failings in the treatment of pancreatic cancer is a lack of curative therapeutics. In addition, local immunosuppression has rendered PDA refractory to immunotherapy. Current chemotherapy combinations can extend survival and the increase quality of life for some patients; however, most do not respond or rapidly acquire resistance. Consequently, improvement of chemotherapy combinations represents the approach most immediately translatable to the clinical benefit of current PDA patients.
Current first-line chemotherapy treatments rely on metabolite poisons to exert their cytotoxicity. Previous efforts proposed modes of cell-autonomous metabolic reprogramming which counteract chemotherapy. More recently, we and others identified that metabolites released from stromal and immune cells directly modulate the metabolism of chemotherapy. Accordingly, targeting re-wired metabolic pathways can either re-sensitize resistant populations to elimination by chemotherapy and/or prevent the onset of resistance in treatment naïve patients.
Determining how Macrophages Remodel the Tumor Microenvironment and Suppress Cytotoxic T Cells

Several barriers in pancreatic cancer treatment are conferred by the presence of immunosuppressive myeloid cells, which are highly represented in these tumors. This immune suppression, driven largely by myeloid cells, renders PDA refractory to immunotherapy which has proven effective in other solid tumors. Consequently, the elimination or reprogramming of these myeloid cells offers potential avenues to provide a much-needed breakthrough for the treatment of patients with pancreatic cancer.
We are working to interrupt the metabolic mechanisms driving immune suppression in the belief they will sensitize pancreatic cancer to immunotherapy. We are working to identify core metabolic pathways in myeloid cells programmed by pancreatic cancer cells and the requirement of these programs for functionality, and determining the metabolic mechanisms by which these myeloid cells mediate immune suppression
Investigating Asparagine Metabolism as a Clinical Target in Pancreatic Cancer

Approaches to target the altered metabolism in pancreatic cancer required to drive unchecked proliferation have mostly been met with modest success. Our previous work demonstrates extracellular asparagine supports pancreatic cancer proliferation when mitochondrial respiration is impaired, and degradation of this pool potently enhances the anti-tumor effect of electron transport chain inhibitors.
Mitochondrial metabolism in tumor-associated macrophages (TAMs) also drives the immune suppressive nature of the tumor microenvironment. We are looking to identify the metabolic programming which enables certain pancreatic cancer cells to produce and release asparagine and understand the mechanism by which asparagine supports mitochondrial metabolism. Conversely, we are examining the impact of mitochondrial targeting combined with asparagine depletion on malignant, stromal, and immune compartments of the tumor microenvironment.
Linking Cellular Plasticity to Inflammation, Chronic Pancreatic Disease, and Tissue Regeneration
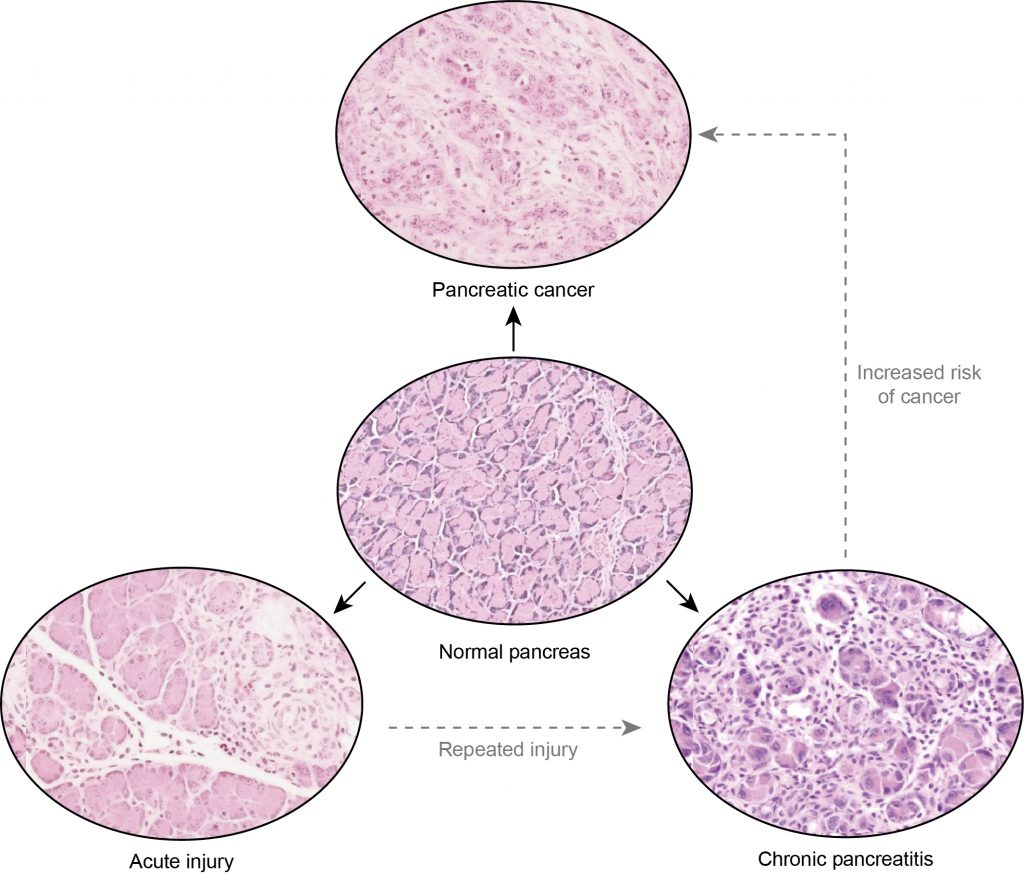
The pancreas lacks a facultative stem cell compartment, and organ homeostasis is maintained by a reversion of terminally differentiated cells to a proliferative progenitor state. This requires the activation of the MAPK pathway, with is hijacked by mutations the oncogene Kras found in almost all pancreatic cancers. We are interested in tracking the signaling and metabolic changes accompanying the de-differentiation process, and its resulting activation of stromal and immune compartments to initiate a wound-healing response that is uncoupled in tumorigenesis.

