Our work falls into three broad categories:
Endothelial genes regulating angiogenesis
Tissue Engineering: Microphysiological Systems
Hereditary Hemorrhagic Telangiectasia (HHT)
Endothelial genes regulating angiogenesis
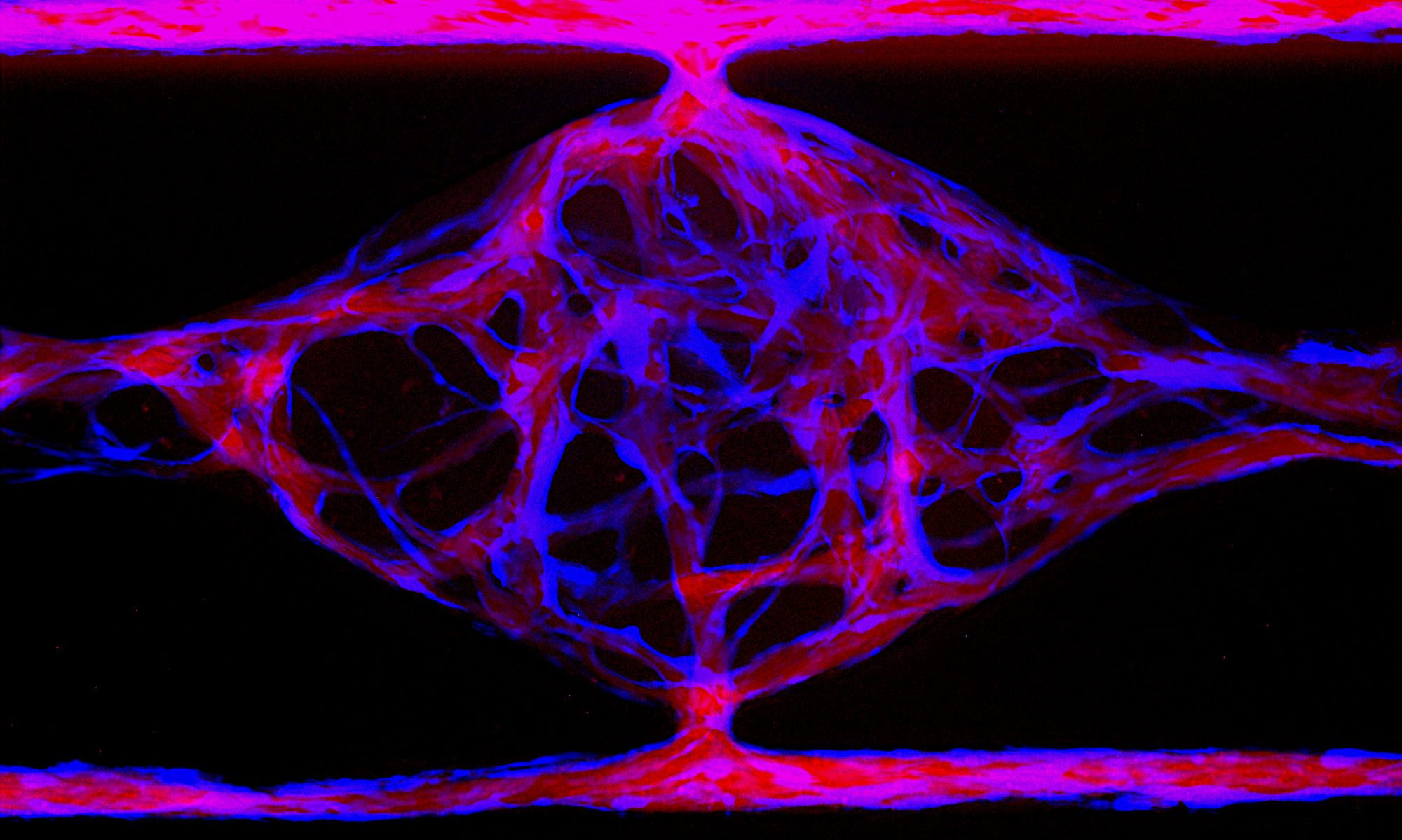
It is our working hypothesis that blood vessels undergoing remodeling and budding to form new vessels, the process of angiogenesis, express novel suites of genes some of which may prove to be useful targets for manipulating blood vessel growth. Furthermore, it is our belief that in vitro systems are useful models for gene discovery and for identifying mechanisms of gene action. We have performed several screens of in vitro systems and have isolated and studied a number of potentially interesting genes.
Current Work
IFITM1 is one of four members of the IFITM family expressed in human. A related family in mouse, called fragilis, is homologous but not orthologous. That is, a single ancestral gene was passed to both the human and mouse lineages, which then duplicated multiple times in each line. Consequently, mouse studies of these genes are not informative as to function of the related genes in human. IFITM1 is expressed in angiogenic EC in human and controls cell-cell interactions that are required for lumen formation. Recent data suggest that IFITM1 regulates EC tight junctions (Popson et al, 2013).
Slug is a member of the snail transcription factor family and regulates epithelial-to-mesenchymal transitions. We have found that slug is upregulated in angiogenic EC, both in vitro and in vivo, and that loss of slug blocks sprouting. MT1-MMP is a matrix metalloproteinase that is critical for EC migration in 3D matrices and loss of MT1-MMP blocks sprouting angiogenesis. We find that MT1-MMP induction requires slug and that sprouting can be restored in slug knockdown cells by re-expression of MT1-MMP (Welch-Reardon et al, 2013).
Fibroblasts are absolutely essential for proper angiogenesis, and in particular provide signals that drive lumen formation. We have found that several of these signals are provided by extracellular matrix proteins, which we have identified in fibroblast-conditioned medium using protein fractionation and mass spectrometry. These include collagen I, beta-Ig-H3, IGFBP7 and SPARC (Newman et al, 2011; Newman et al, 2012).
Relevant lab publications
DTT Phan, RHF Bender, JW Andrejecsk, A Sobrino, and Hughes CCW- Experimental Biology and Medicine, 2017. Blood–brain barrier-on-a-chip: Microphysiological systems that capture the complexity of the blood–central nervous system interface
Newman AC, Nakatsu MN, Chou W, Gershon PD, and Hughes CCW. 2011. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Molecular Biology of the Cell. Molecular
NewmanAC, Chou W, Welch-ReardonKM, FongAH, PopsonSA, PhanDT, SandovalDR, GershonPD, and HughesCCW. 2012. Analysis of stromal cell secretomes reveals a critical role for stromal cell-derived HGF and fibronectin in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 33: 513-22.
Popson SA, Ziegler M, Chen X, Holderfield, MT, Shaaban C, Fong AH, Papkoff J and Hughes CCW. 2013 Interferon-induced transmembrane protein 1 regulates endothelial lumen formation during angiogenesis (submitted).
Welch-Reardon KM, Ehsan SM, Wang K, Newman AC, Romero-Lopez M, Fong AH, George SC, Edwards RA and Hughes CCW. 2013. Angiogenic sprouting is regulated by endothelial cell expression of Slug (Snai2) (submitted)
Previous Work
HESR1 is a bHLH transcription factor that is rapidly uprgeulated in angiogenic EC and regulates both sprouting and tube formation. Expression of HESR1 downregulates the growth factor receptor VEGFR2, thus promoting a more quiescent EC phenotype (Henderson et al 2001).
We further showed that HESR1 is a downstream effector of notch, a transmembrane receptor that in many systems controls cell differentiation. Our data suggest that Notch/HESR1 may be critical regulators of the angiogenic phenotype (Taylor et al 2002). Specifically, our lab was the first to show that knockdown of notch in EC leads to an increase in tip cell number and promotes branching (Sainson et al, 2005). HESR1 appears to act through multiple weak interactions with SP-1 and other factors on promoters driven by initiator elements. A TATA box can override the inhibitory effects of HESR1 on transcription (Holderfield et al 2006). Further work showed that TNF primes EC for sprouting by inducing a tip cell phenotype (Sainson et al, 2008), including upregulation of jagged-1 (Johnston et al, 2009).
Relevant lab publications
1. Henderson AM, Wang S-J, Taylor AC, Aitkenhead M and Hughes CCW 2001 The bHLH transcription factor HESR1 regulates endothelial cell tube formation. J. Biol. Chem. 276: 6169-6176
2. Aitkenhead M, Wang S-J, Mestas J, Heard C and Hughes CCW. 2002. Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: upregulation of ESM-1, big-h3 and NrCAM. Microvascular Research. 63: 159-171
3. Taylor KL and Hughes CCW. 2002. Notch activation during endothelial cell tube formation in vitro targets the basic HLH transcription factor HESR1 and downregulates VEGFR-2/KDR expression. Microvascular Research. 64: 372-383
4. Nakatsu MN, Sainson RCA, Aoto JN, Taylor KL, Aitkenhead M, Pérez del Pulgar S, Carpenter PM, and Hughes CCW. 2003 Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvascular Research 66: 102-112 [MVR most downloaded article for 2003]
5. Nakatsu MN, Sainson RCA, Pérez del Pulgar S, Aoto JN, Aitkenhead M, Taylor KL, Carpenter PM and Hughes CCW. 2003 VEGF121 and VEGF165 regulate blood vessel diameter through VEGFR-2 in an in vitro angiogenesis model. Laboratory Investigation 83: 1873-1885.
6. Sainson RCA, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, and Hughes CCW. 2005 Cell autonomous Notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J 19: 1027-9
7. Holderfield MT, Henderson Anderson AM, Kokubo H, Chin MT, Johnson RL and Hughes CCW. 2006 HESR1/CHF2 suppresses VEGFR2 transcription independent of binding to E-boxes. Biochem. Biophys. Res. Comm. 346: 637-648
8. Nakatsu MN, Davis J, and Hughes CCW 2007 An optimized fibrin gel bead assay for the study of angiogenesis in vitro. J. Vis. Exp. (JoVE) 3: (Apr) doi:10.3791/186
9. Sainson RCA, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, and Hughes CCW. 2008 TNF Primes Endothelial Cells for Angiogenic Sprouting by Inducing a Tip Cell Phenotype. Blood 111: 4997-5007.
10. Johnston DA, Dong B, and Hughes CCW. 2009 TNF induction of jagged-1 in endothelial cells is NFkB dependent. Gene 435: 36-44.
Tissue Engineering: Microphysiological Systems