Molecules to be radiolabeled are identified based on the binding and selectivity of the small molecule to the molecular targets and/or biomarker. Generally speaking, design of a suitable small molecule takes into consideration the ability to readily and rapidly radiolabel the molecule with radioisotopes such as carbon-11, fluorine-18, iodine-123. Automated radiosynthesis units are used to develop the radiolabeling methods, develop procedures of purification and quality control and assurance is validated as in 18F-fallypride and 11C-fallypride (DOE support). A standard operating procedure (SOP) for the particular radiopharmaceutical is developed. Subsequently the radiopharmaceutical is taken to in vitro and in vivo preclinical testing.
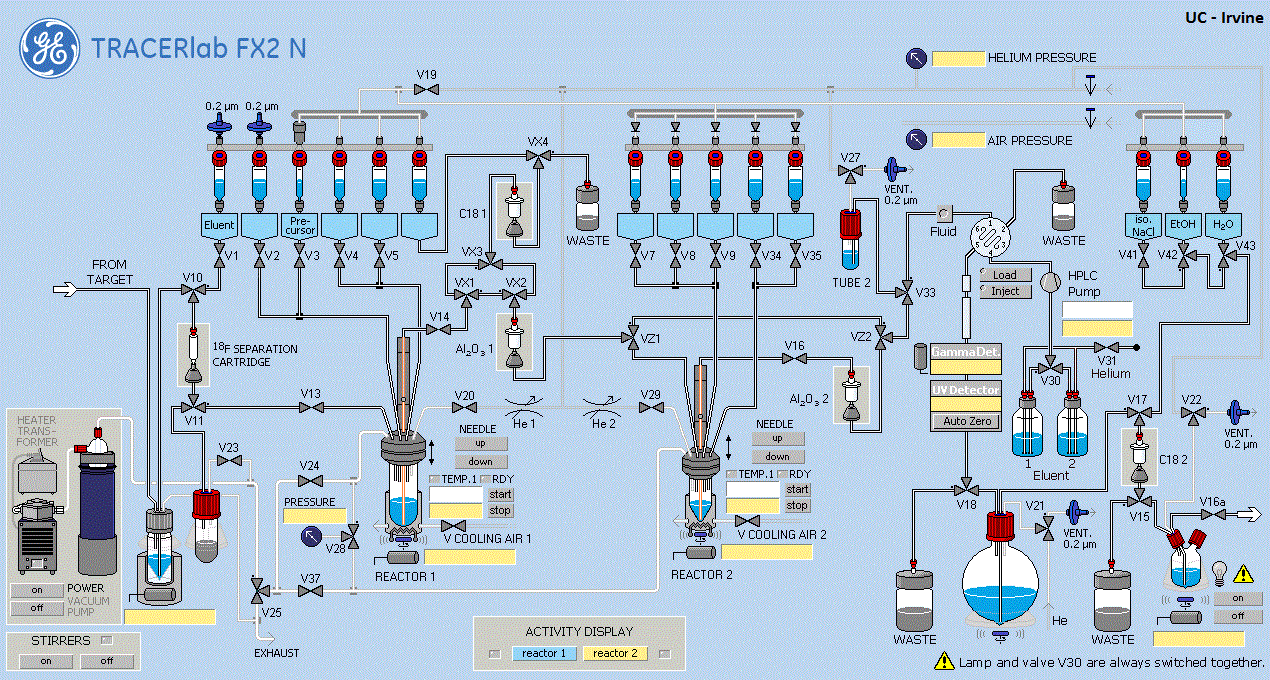
The Radiochemistry laboratory in Preclinical Imaging, Radiological Sciences has been operational since 2018. It is located in Medical Sciences and has all the facilities of hot cells, automated synthesizers, radioactivity measurement equipment, high performance liquid chromatographic systems, quality control equipment, radioactive thin layer chromatography, gas chromatography, optiquant imaging system for autoradiographic this layer chromatography, certified fume hood, dedicated radioiodination hoods and other equipment. The facility has a dedicated, computerized radiation monitoring filtration and exhaust system in place.
 Von Gahlen Hot Cells and GE Automated Synthesizers
Von Gahlen Hot Cells and GE Automated Synthesizers