Cytochrome P450
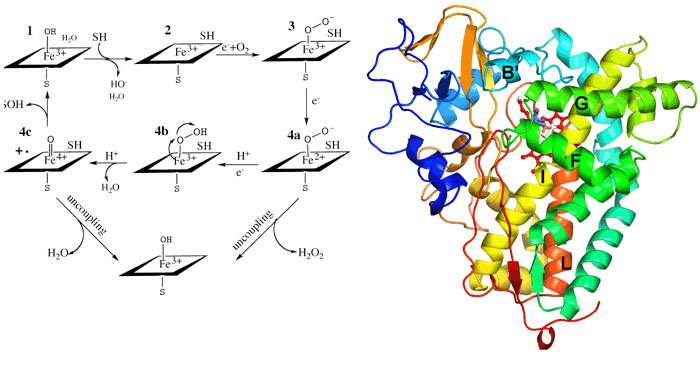
Cytochromes P450 occur throughout the biosphere and since the early 1980s,hundreds of different P450 genes have been identified making this one of the most widespread and diverse biocatalysts. P450s participate in the metabolism of sex hormones, vitamin D, and bile acids in mammals ecdysones in insects and terpenes in plants. P450 monooxygenase systems also participate in the metabolism of a variety of drugs and other xenobiotic substances and provide one of the primary means by which the body rids itself of toxic substances. P450s also participate in the conversion of aromatic hydrocarbons into potent carcinogens. Moreover, P450s are the target for certain classes of commercially useful antifungal, agrochemical, and therapeutic agents. P450s are single polypeptide chains ranging in size from 40-50Kda and contain a single heme group coordinated to a Cys residue. The enzyme binds substrate, SH, which displaces a water molecule coordinated to the heme iron. This results in an easily detected spectral shift owing to the change of the iron spin state from hexacoordinate low-spin to pentacoordinate high-spin. Reduction of the heme iron followed by oxygenation and a second electron transfer step leads to O-Obond cleavage and formation of the hypothetical Fe(IV)=O intermediate. The Fe(IV)=O intermediate, or its electronic equivalent, removes an H atom from the substrate followed by formation of the S-OH bond. The electron transferreactions involve complex formation between an electron donor protein and the P450. In microsomal P450s the electron donor is P450 reductase which contains FMN and FAD. Electrons are funneled from NADPH to the flavins and finally to the P450 heme iron. Our studies with P450s involve crystallography, protein engineering, and spectroscopy. Most recently we have been focussing on P450 electron transfer complexes.