Nitric Oxide Synthase
Nitric oxide synthase or NOS catalyzes the conversion of L arginine to nitric oxide (NO) and citrulline. NO is an important signaling molecule involved in neural transmission, smooth muscle relaxation, and the immune response. There are 3 main human NOS isoforms: inducible NOS or iNOS generates NO during the immune response where NO acts as a cytotoxic molecule; endothelial NOS or eNOS produces NO for vasodilatation and; neuronal NOS or nNOS produces NO involved in neuronal physiology. A unique feature of NOS is the requirement for tetrahydrobipterin which serves an electron transfer function. NOS is a complex molecule that contains a heme domain and FMN/FAD-containing flavin domain. The flavin domain delivers NADPH-derived electrons to the heme domain where the heme iron is reduced leading to oxygen activation and arginine oxidation. Both eNOS and nNOS are regulated by the binding of calmodulin to the linker region that joins the heme and flavin domains.
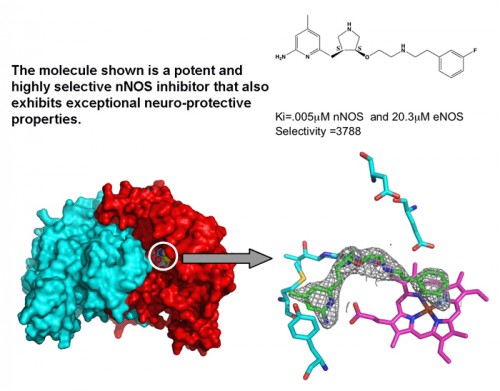
The over production of NO by nNOS is associated with a number of neurodegenerative disorders. As a result specifically blocking nNOS can provide nueuroprotection in diseases associated with NO over production. However, eNOS is critical for maintianing proper blood pressure and thus, any non-selective NOS drug will lead to hypertension. The goal is to develop drugs that are highly selective to nNOS. In collaboration with Rick Silverman at Northwestern, a combination of crystallography, protein engineering, computer modeling, and organic synthesis have been used to develop the potent nNOS-selective inhibitors.