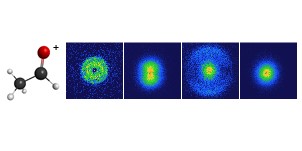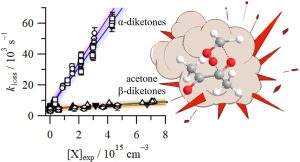
Our new paper describing the kinetics of reactions of CH2OO with ketones and diketones has been published in the Journal of Physical Chemistry A. We find rate constants for the β-diketone reactions that are similar to that of acetone, while those for the α-diketones are around 30 times larger. The trends are interpreted using frontier molecular orbital theory.
You can read the paper here: 10.1021/acs.jpca.1c05280









 We have been awarded $394,638 by the Chemistry Division of the National Science Foundation for a three-year project titled, “Kinetic and Mechanistic Studies of Criegee Intermediate reactivity.” The project is part of the Environmental Chemical Sciences program. We will use laser flash photolysis and broadband transient absorption spectroscopy to measure temperature-dependent rate constants of Criegee intermediate reactions with trace atmospheric gases. Specifically, we will study Criegee intermediate reactions with multifunctional volatile organic compounds, radical species, and explore the influence of humidity on reactivity. A new outreach program will establish air quality and weather monitoring stations at local high schools.
We have been awarded $394,638 by the Chemistry Division of the National Science Foundation for a three-year project titled, “Kinetic and Mechanistic Studies of Criegee Intermediate reactivity.” The project is part of the Environmental Chemical Sciences program. We will use laser flash photolysis and broadband transient absorption spectroscopy to measure temperature-dependent rate constants of Criegee intermediate reactions with trace atmospheric gases. Specifically, we will study Criegee intermediate reactions with multifunctional volatile organic compounds, radical species, and explore the influence of humidity on reactivity. A new outreach program will establish air quality and weather monitoring stations at local high schools.