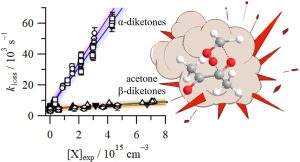
Our new paper describing the kinetics of reactions of CH2OO with ketones and diketones has been published in the Journal of Physical Chemistry A. We find rate constants for the β-diketone reactions that are similar to that of acetone, while those for the α-diketones are around 30 times larger. The trends are interpreted using frontier molecular orbital theory.
You can read the paper here: 10.1021/acs.jpca.1c05280
