 Congratulations to Morgan Coburn on a successful Ph.D. Dissertation Defense “Using Chimeric Models to Study the Interactions between Human Microglia and Alzheimer’s Disease Pathology in vivo.“
Congratulations to Morgan Coburn on a successful Ph.D. Dissertation Defense “Using Chimeric Models to Study the Interactions between Human Microglia and Alzheimer’s Disease Pathology in vivo.“
Amanda McQuade Ph.D. Dissertation Defense
Congratulations to Amanda McQuade on a successful Ph.D. Dissertation Defense Development and application of a human microglia model to examine the influence of genetic risk factors on microglial function in Alzheimer’s disease:
Alzheimer’s disease (AD) is a progressive neurodegenerative disease for which there is no cure. Worldwide, AD is estimated to affect 50 million people with 10 million new cases each year. Developing therapeutics for Alzheimer’s disease has been particularly difficult given the complex etiology of this disease. Alzheimer’s disease is characterized by an accumulation of parenchymal beta-amyloid protein plaques, tau neurofibrillary tangles, and neuroinflammation. For sporadic AD, which accounts for around 95 % of AD cases, the direct trigger of neurodegeneration remains unclear. Understanding the mechanistic pathophysiology of this disease will allow for the development of more effective targeted therapeutics and biomarker studies to help patients.
In the last decade, genome-wide association studies (GWAS) have renewed interest in neuroinflammation as a potential disease-modifying mechanism. These large-scale genetic studies have uncovered a striking enrichment of immune-specific genes as risk-factors for Alzheimer’s disease. However, the function of many of these GWAS loci are not well understood. Additionally, many of these genes have poor homology between human cells and traditional murine disease-models. Thus, there is a critical need to develop a model of human microglia in order to understand how these immune risk factors influence human microglial function.
The focus of this dissertation is to develop and characterize a model of human microglia that can be readily studied without the need for isolation of human brain tissue surgically or post-mortem. Building on previous research in the lab, we have developed a model of human microglia differentiated from induced pluripotent stem cells (iPS-Microglia). This model follows developmental ontogeny with a primary differentiation into CD43+ hematopoietic progenitor cells before transition into a microglial differentiation medium containing neuron and astrocyte
derived cytokines to educate our microglia in a homeostatic brain-like environment. The result is a highly pure population of iPS-Microglia which perform key microglial functions and cluster alongside human microglial transcriptomes (Chapter 1).
Because this microglial model is fully defined beginning from iPS cells, it is possible to combine this approach with modern molecular biological manipulation such as CRISPR gene editing. This dissertation focuses on studies surrounding the AD-risk loci Triggering Receptor Expressed on Myeloid Cells II (TREM2). Predicted loss of function mutations in TREM2 increase Alzheimer’s disease risk up to 2-3 fold making it the highest microglial-specific risk factor for AD. By performing CRISPR-mediated knockout of TREM2 in human iPSCs and differentiating into
microglia, we find that TREM2-knockout locks microglia in a homeostatic state. Specifically, microglia lacking TREM2 are deficient in SYK-mediated phagocytosis, CXCR4-mediated migration, and are more sensitive to MCSF-mediated cell death.
In vivo, this translates to an inability to perform chemotaxis towards and compaction of beta-amyloid plaques leading to a build-up of pathology in the brain. We also characterize a distinct lack of transcriptional activation in TREM2 knockout cells suggesting that induction of the Disease Associated Microglia (DAM) profile may be an essential immune response in shielding the brain from dementia (Chapter 2).
Because TREM2-knockout cells are locked in a homeostatic state, they express high levels of homeostatic microglia markers P2RY12 and P2RY13. These purinergic receptors are critical for microglial communication with neurons and clearance of dead cells. We show that purinergic signaling has a profound effect on microglial motility and process extension and that hyper-expression and response of purinergic signaling in TREM2-knockout microglia may render these cells unable to sense gradients and activate against disease pathology. Furthermore, we highlight that partial inhibition of purinergic receptors will rescue migratory deficits characterized in TREM2-knockout microglia suggesting a therapeutic mechanism (Chapter 3).
Taken as a whole, the development of the iPS-Microglia model has yielded critical insight into the interaction of microglial function and the development of Alzheimer’s disease. This model has been readily adopted by many academic labs as well as pharmaceutical companies with the aim of studying human microglial function or other disease risk loci. By further studying AD risk genes in this fashion, we may be able to converge on several mechanisms by which microglial functions are able to attenuate or drive neurodegeneration. Focusing therapeutic efforts on these pathways could lead to the development of immunotherapies for Alzheimer’s disease.
Claes et al. publish in Molecular Neurodegeneration
Plaque-associated human microglia accumulate lipid droplets in a chimeric model of Alzheimer’s disease
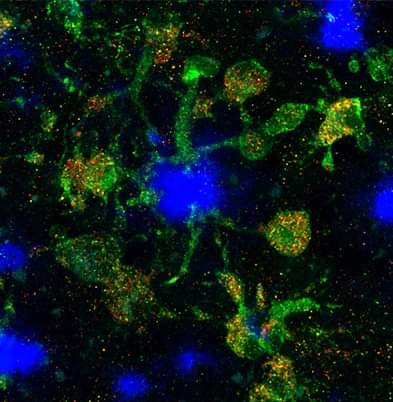 Background: Disease-associated microglia (DAMs), that surround beta-amyloid plaques, represent a transcriptionally-distinct microglial profile in Alzheimer’s disease (AD). Activation of DAMs is dependent on triggering receptor expressed on myeloid cells 2 (TREM2) in mouse models and the AD TREM2-R47H risk variant reduces microglial activation and plaque association in human carriers. Interestingly, TREM2 has also been identified as a microglial lipid-sensor, and recent data indicates lipid droplet accumulation in aged microglia, that is in turn associated with a dysfunctional proinflammatory phenotype. However, whether lipid droplets (LDs) are present in human microglia in AD and how the R47H mutation affects this remains unknown.
Background: Disease-associated microglia (DAMs), that surround beta-amyloid plaques, represent a transcriptionally-distinct microglial profile in Alzheimer’s disease (AD). Activation of DAMs is dependent on triggering receptor expressed on myeloid cells 2 (TREM2) in mouse models and the AD TREM2-R47H risk variant reduces microglial activation and plaque association in human carriers. Interestingly, TREM2 has also been identified as a microglial lipid-sensor, and recent data indicates lipid droplet accumulation in aged microglia, that is in turn associated with a dysfunctional proinflammatory phenotype. However, whether lipid droplets (LDs) are present in human microglia in AD and how the R47H mutation affects this remains unknown.
Methods: To determine the impact of the TREM2 R47H mutation on human microglial function in vivo, we transplanted wild-type and isogenic TREM2-R47H iPSC-derived microglial progenitors into our recently developed chimeric Alzheimer mouse model. At 7 months of age scRNA-seq and histological analyses were performed.
Results: Here we report that the transcriptome of human wild-type TREM2 and isogenic TREM2-R47H DAM xenografted microglia (xMGs), isolated from chimeric AD mice, closely resembles that of human atherosclerotic foam cells. In addition, much like foam cells, plaque-bound xMGs are highly enriched in lipid droplets. Somewhat surprisingly and in contrast to a recent in vitro study, TREM2-R47H mutant xMGs exhibit an overall reduction in the accumulation of lipid droplets in vivo. Notably, TREM2-R47H xMGs also show overall reduced reactivity to plaques, including diminished plaque-proximity, reduced CD9 expression, and lower secretion of plaque-associated APOE.
Conclusions: Altogether, these results indicate lipid droplet accumulation occurs in human DAM xMGs in AD, but is reduced in TREM2-R47H DAM xMGs, as it occurs secondary to TREM2-mediated changes in plaque proximity and reactivity.
Jessica Sanchez Awarded UCI Summer Inclusive Excellence Grant Award 2021
Grants are awarded to students whose research, teaching, and/or service supports diversity, equity, and inclusion and contributes to UCI’s effort to open opportunities for under-represented groups in higher education.
Amanda McQuade receives the 2021 James L. McGaugh Award
 Amanda McQuade receives the 2021 James L. McGaugh Award for her publication studying TREM2 in human iPS-Microglia: https://www.nature.com/
Amanda McQuade receives the 2021 James L. McGaugh Award for her publication studying TREM2 in human iPS-Microglia: https://www.nature.com/
Morgan Coburn recognized as “Most Promising Future Faculty”
 Congratulations to Angeline Duke and Morgan Coburn for winning the 2021 Most Promising Future Faculty Award! The Most Promising Future Faculty Award recognizes graduate students who show great promise through their effectiveness as instructors or teaching assistants.
Congratulations to Angeline Duke and Morgan Coburn for winning the 2021 Most Promising Future Faculty Award! The Most Promising Future Faculty Award recognizes graduate students who show great promise through their effectiveness as instructors or teaching assistants.
Morgan Coburn is currently a Ph.D. candidate in the Department of Neurobiology and Behavior. Her teaching experience is off the charts, having served in ten different teaching capacities. Her research focuses on the action of microglia, the innate immune cell of the brain, and their role in Alzheimer’s disease. Morgan hopes to find a cure for one of society’s most heartbreaking diseases and she wants to give back and teach full time one day.
Congratulation once again to Morgan Coburn for winning the 2021 Most Promising Future Faculty Award!
Amanda McQuade awarded at 12th Annual Emerging Scientists Symposium
This prize is awarded in honor of Dr. Carl W. Cotman’s legacy in scientific accomplishments throughout his Directorship of the Institute for Memory Impairments and Neurological Disorders and the development of the health sciences at UCI. Dr. Cotman’s passion and longstanding interest in the brain have fostered research within the Institute and on the UCI campus throughout the years. The recipient of this award is selected from those who have excelled in their studies on memory impairments and neurological disorders and have helped to advance the mission of the Institute. You should be proud of your accomplishments and for having received this award.
Amanda McQuade awarded the Junior Faculty Award
Congratulations to Amanda McQuade for winning a Junior Faculty Award for the 15th International Conference on Alzheimer’s and Parkinson’s disease. The Organizing Committee of AD/PD™ 2021 recognized the top junior and trainee abstract presenters with Junior Faculty Awards. Amanda was recognized for her talk “INTERACTIONS BETWEEN ALZHEIMER’S RISK FACTORS TREM2 AND MS4A6A IN HUMAN IPS-MICROGLIA”.
Modeling Alzheimer’s risk using human TREM2-knockout microglia
Amanda McQuade from Dr. Mathew Blurton-Jones’s lab discusses her protocol for differentiating microglia from induced pluripotent stem cells (iPSCs) and the use of these microglia in vivo and in vitro to uncover the mechanisms of immune activation and neurodegeneration in Alzheimer’s disease. Based on the 2020 Nature Communications paper “Gene Expression and Functional Deficits Underlie TREM2-Knockout Microglia Responses in Human Models of Alzheimer’s Disease“.