The Green group uses a mixture of theory and experiment to elucidate fundamental aspects of metalloprotein chemistry. We are particularly interested in understanding the factors that govern biological C-H bond activation. One system of interest is a class of proteins called cytochromes P450. These thiolate-ligated heme proteins play critical roles in xenobiotic metabolism and steroid biosynthesis, and they have been implicated in carcinogenesis. Our interest in P450s stems not only from their biological importance but also from a desire to harness their synthetic potential: P450s catalyze the selective oxidation of inert C-H bonds—A Holy Grail of chemical synthesis—Research in the Green lab examines two rather enigmatic aspects of P450 chemistry: 1) the enzyme’s use of an electron-rich thiolate-ligated heme to catalyze the oxidation of inert hydrocarbons and 2) the enzyme’s ability to perform these demanding oxidations without damage to its own relatively fragile protein superstructure.
Below are some of our most exciting discoveries in this area:
1) Ascorbate Peroxidase Compound II Is an Iron (IV) Oxo Species [link]
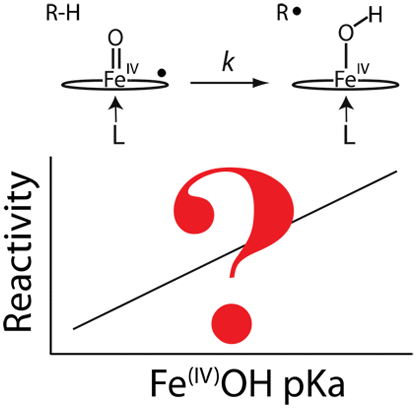 The protonation state of the iron(IV) oxo (or ferryl) form of ascorbate peroxidase compound II (APX-II) is a subject of debate. It has been reported that this intermediate is best described as an iron(IV) hydroxide species. Neutron diffraction data obtained from putative APX-II crystals indicate a protonated oxygenic ligand at 1.88 Å from the heme iron. This finding, if correct, would be unprecedented. A basic iron(IV) oxo species has yet to be spectroscopically observed in a histidine-ligated heme enzyme. The importance of ferryl basicity lies in its connection to our fundamental understanding of C–H bond activation. Basic ferryl species have been proposed to facilitate the oxidation of inert C–H bonds, reactions that are unknown for histidine-ligated hemes enzymes. To provide further insight into the protonation status of APX-II, we examined the intermediate using a combination of Mössbauer and X-ray absorption spectroscopies. Our data indicate that APX-II is an iron(IV) oxo species with an Fe–O bond distance of 1.68 Å, a K-edge pre-edge absorption of 18 units, and Mössbauer parameters of ΔEq = 1.65 mm/s and δ = 0.03 mm/s. APX, thus, appears to be a typical histidine-ligated peroxidase, possessing a compound II species with an acidic pKa, in contrast to the much more basic P450-II (pKa ≈ 12.0).
The protonation state of the iron(IV) oxo (or ferryl) form of ascorbate peroxidase compound II (APX-II) is a subject of debate. It has been reported that this intermediate is best described as an iron(IV) hydroxide species. Neutron diffraction data obtained from putative APX-II crystals indicate a protonated oxygenic ligand at 1.88 Å from the heme iron. This finding, if correct, would be unprecedented. A basic iron(IV) oxo species has yet to be spectroscopically observed in a histidine-ligated heme enzyme. The importance of ferryl basicity lies in its connection to our fundamental understanding of C–H bond activation. Basic ferryl species have been proposed to facilitate the oxidation of inert C–H bonds, reactions that are unknown for histidine-ligated hemes enzymes. To provide further insight into the protonation status of APX-II, we examined the intermediate using a combination of Mössbauer and X-ray absorption spectroscopies. Our data indicate that APX-II is an iron(IV) oxo species with an Fe–O bond distance of 1.68 Å, a K-edge pre-edge absorption of 18 units, and Mössbauer parameters of ΔEq = 1.65 mm/s and δ = 0.03 mm/s. APX, thus, appears to be a typical histidine-ligated peroxidase, possessing a compound II species with an acidic pKa, in contrast to the much more basic P450-II (pKa ≈ 12.0).
2) Reduction Potentials of P450 Compounds I and II: Insight into the Thermodynamics of C−H Bond Activation [link]
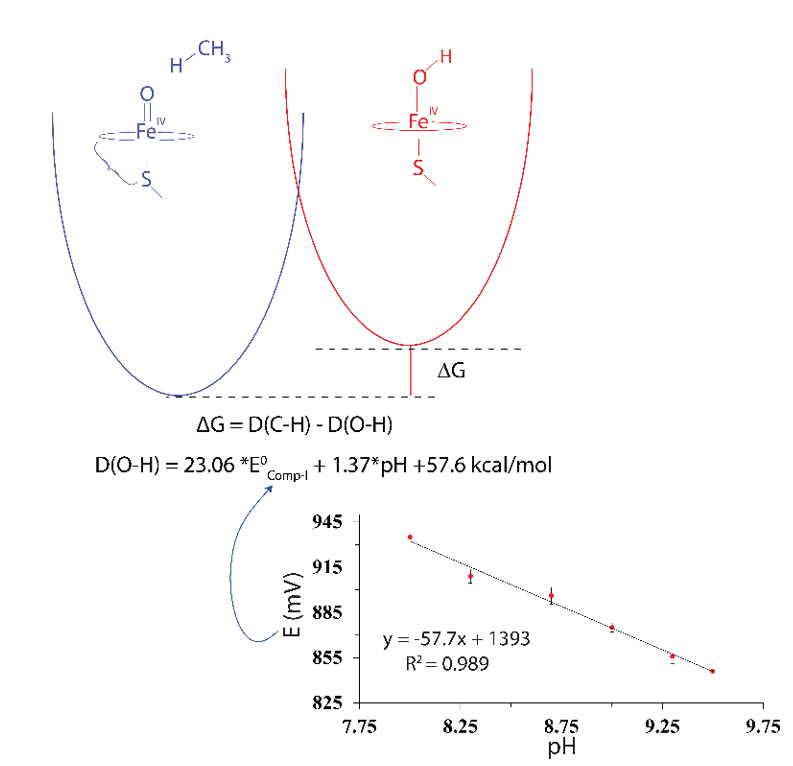 We present a mixed experimental/theoretical determination of the bond strengths and redox potentials that define the ground-state thermodynamics for C−H bond activation in cytochrome P450 catalysis. Using redox titrations with [Ir(IV)Cl 6 ] 2− , we have determined the compound II/ferric (or Fe(IV)OH/Fe(III)OH 2 ) couple and its associated D(O−H)Ferric bond strength in CYP158. Knowledge of this potential as well as the compound II/ ferric (Fe(IV)O/Fe(III)OH) reduction potential in horseradish peroxidase and the two electron compound I/ ferric (or Fe(IV)O(Por•)/Fe(III)OH 2 (Por)) reduction potential in aromatic peroxidase has allowed us to gauge the accuracy of theoretically determined bond strengths. Using the restricted open shell (ROS) method as proposed by Wright and coworkers, we have obtained O−H bond strengths and associated redox potentials for charge neutral H-atom reductions of these iron (IV)-hydroxo and -oxo porphyrin species that are within 1 kcal/mol of experimentally determined values, suggesting that the ROS method may provide accurate values for the P450-II O−H bond strength and P450-I reduction potential. The efforts detailed here indicate that the ground-state thermodynamics of C−H bond activation in P450 are best described as follows: E0′s]CompI = 1.22 V (at pH 7, vs NHE) with D(O−H)CompII = 95 kcal/mol and E0′CompII = 0.99 V (at pH 7, vs NHE) with D(O−H)Ferric = 90 kcal/mol.
We present a mixed experimental/theoretical determination of the bond strengths and redox potentials that define the ground-state thermodynamics for C−H bond activation in cytochrome P450 catalysis. Using redox titrations with [Ir(IV)Cl 6 ] 2− , we have determined the compound II/ferric (or Fe(IV)OH/Fe(III)OH 2 ) couple and its associated D(O−H)Ferric bond strength in CYP158. Knowledge of this potential as well as the compound II/ ferric (Fe(IV)O/Fe(III)OH) reduction potential in horseradish peroxidase and the two electron compound I/ ferric (or Fe(IV)O(Por•)/Fe(III)OH 2 (Por)) reduction potential in aromatic peroxidase has allowed us to gauge the accuracy of theoretically determined bond strengths. Using the restricted open shell (ROS) method as proposed by Wright and coworkers, we have obtained O−H bond strengths and associated redox potentials for charge neutral H-atom reductions of these iron (IV)-hydroxo and -oxo porphyrin species that are within 1 kcal/mol of experimentally determined values, suggesting that the ROS method may provide accurate values for the P450-II O−H bond strength and P450-I reduction potential. The efforts detailed here indicate that the ground-state thermodynamics of C−H bond activation in P450 are best described as follows: E0′s]CompI = 1.22 V (at pH 7, vs NHE) with D(O−H)CompII = 95 kcal/mol and E0′CompII = 0.99 V (at pH 7, vs NHE) with D(O−H)Ferric = 90 kcal/mol.
3) Iron(IV)hydroxide pKa and the Role of Thiolate Ligation in C-H Bond Activation by Cytochrome P450 [link]
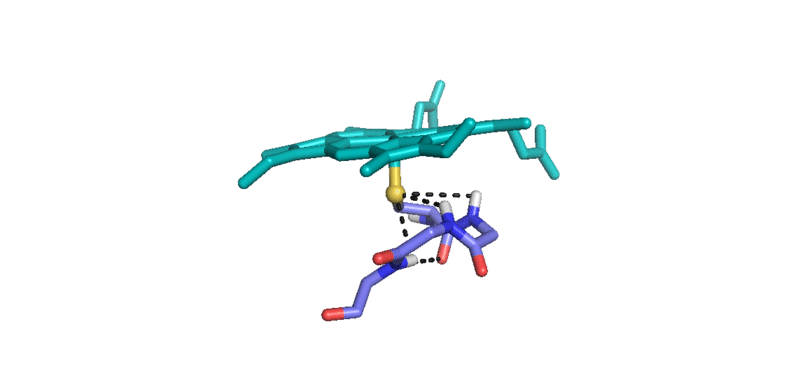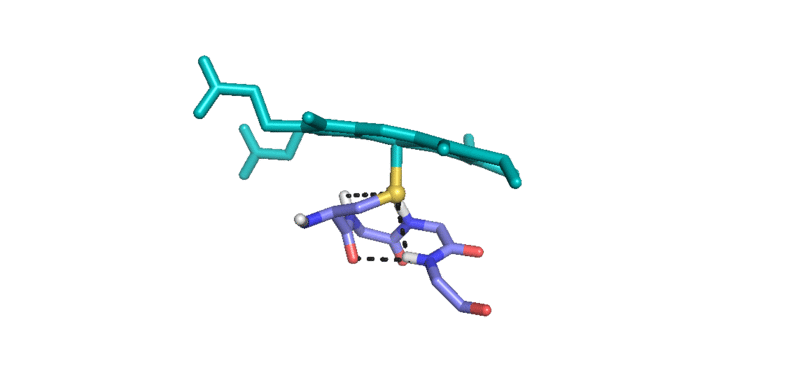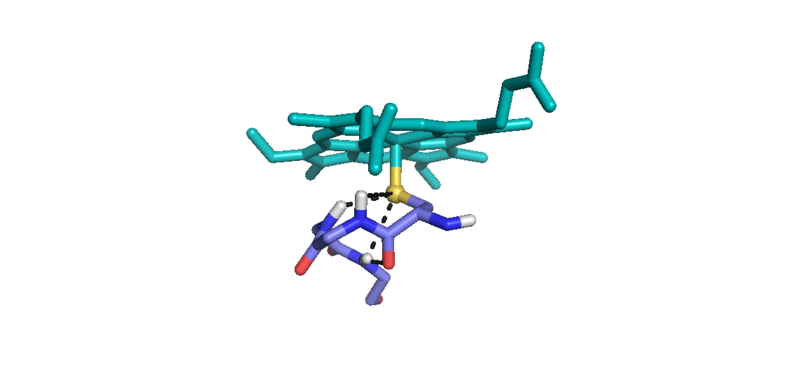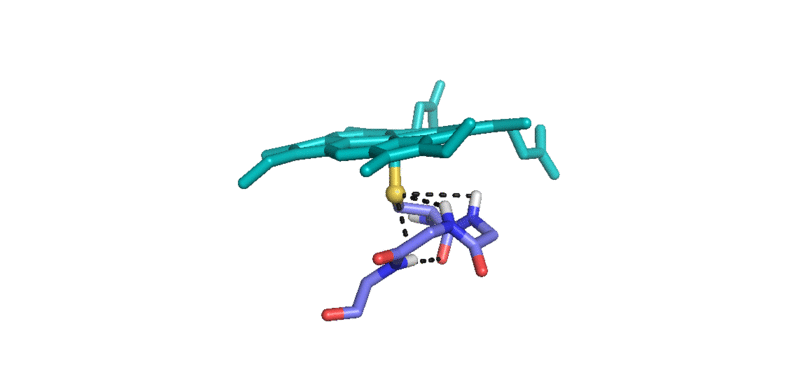
 This paper provides a new paradigm for understanding biological C-H bond activation. At its heart lies an exploration of the factors that govern the partition between productive C-H bond activation and non-productive oxidations of the protein superstructure. Nature’s ability to suppress energetically favorable non-productive pathways is an enigmatic aspect of biological C-H bond activation—How does an enzyme oxidize one of the most inert chemical bonds without damaging itself in the process?—We examine this issue through the lens of P450 catalysis. The centerpiece of the work is the measurement of the ferryl pKa of P450 compound II. The determination of this thermodynamic parameter is a remarkable achievement (the first of its kind). Its value provides the first quantitative measure of the thiolate ligand’s impact on P450 catalysis, and it holds the key to understanding Nature’s ability to suppress nonproductive oxidations during C-H bond activation. Knowledge of this quantity has allowed us to develop novel thermodynamic and kinetic analyses that reveal how the relative free energy (for the productive and non-productive pathways) as well as the rate constants for non-productive oxidations depend on the ferryl pKa. Our results provide fundamental insight into biological C-H bond activation as well as a blueprint for protein/catalyst design.
This paper provides a new paradigm for understanding biological C-H bond activation. At its heart lies an exploration of the factors that govern the partition between productive C-H bond activation and non-productive oxidations of the protein superstructure. Nature’s ability to suppress energetically favorable non-productive pathways is an enigmatic aspect of biological C-H bond activation—How does an enzyme oxidize one of the most inert chemical bonds without damaging itself in the process?—We examine this issue through the lens of P450 catalysis. The centerpiece of the work is the measurement of the ferryl pKa of P450 compound II. The determination of this thermodynamic parameter is a remarkable achievement (the first of its kind). Its value provides the first quantitative measure of the thiolate ligand’s impact on P450 catalysis, and it holds the key to understanding Nature’s ability to suppress nonproductive oxidations during C-H bond activation. Knowledge of this quantity has allowed us to develop novel thermodynamic and kinetic analyses that reveal how the relative free energy (for the productive and non-productive pathways) as well as the rate constants for non-productive oxidations depend on the ferryl pKa. Our results provide fundamental insight into biological C-H bond activation as well as a blueprint for protein/catalyst design.
This work was highlighted in the following:
1) “Mysterious selectivity of nature’s blowtorches solved”, Urquhart, J. Chemistry World. Nov. 14, 2013. 2) “Enzymatic C-H bond activation: Using push to get pull”, Groves, J.T. Nature Chemistry, 2014, 6, 89-91. 3) “1958–2014: After 56 Years of Research, Cytochrome P450 Reactivity Is Finally Explained”, McQuarters, A.B.; Wolf, M.W.; Hunt, A.P., and Lehnert, N. Angew. Chem. Int. Ed., 2014, 53, 4750-4752.
4) Cytochrome P450 Compound I: Capture, Characterization, and C-H Bond Activation Kinetics [link]
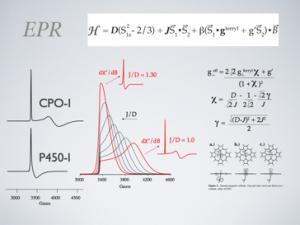 When we started working in the field, most of the intermediates in the P450 catalytic cycle had been catalogued, but compound I, the high valent species believed to be directly involved in substrate oxidations, remained elusive. So elusive, in fact, that the intermediate obtained an almost mythical status in the field. Indeed, a review on the subject as recent as 2010 was entitled “The Mystery of Cytochrome P450 Compound I”. For almost four decades, the capture and characterization of this intermediate stood as an unattainable goal in biological chemistry. Using highly purified protein and rapid mixing techniques, we were able to prepare the intermediate in high yield, allowing for detailed spectroscopic and kinetic characterizations. This work not only verified the existence of P450 compound I, bu it also confirmed the rebound mechanism of substrate hydroxylations.
When we started working in the field, most of the intermediates in the P450 catalytic cycle had been catalogued, but compound I, the high valent species believed to be directly involved in substrate oxidations, remained elusive. So elusive, in fact, that the intermediate obtained an almost mythical status in the field. Indeed, a review on the subject as recent as 2010 was entitled “The Mystery of Cytochrome P450 Compound I”. For almost four decades, the capture and characterization of this intermediate stood as an unattainable goal in biological chemistry. Using highly purified protein and rapid mixing techniques, we were able to prepare the intermediate in high yield, allowing for detailed spectroscopic and kinetic characterizations. This work not only verified the existence of P450 compound I, bu it also confirmed the rebound mechanism of substrate hydroxylations.
This work was highlighted in the following journals:
1) “Glimpsing the Critical Intermediate in Cytochrome P450 Oxidations”, Sligar, S.G. Science, 2010, 330, 924-925. 2) “P450 Compound I, Finally”, Sheppard, T.L. Nature Chemical Biology, 2011, 7, 2. 3) “Cytochrome P450: Caught in the act”, Nature Chemistry, 2011, 3, 92. 4) “1958–2014: After 56 Years of Research, Cytochrome P450 Reactivity Is Finally Explained”, McQuarters, A.B.; Wolf, M.W.; Hunt, A.P., and Lehnert, N. Angew. Chem. Int. Ed., 2014, 53, 4750-4752.
This work was highlighted in the news from several organizations:
1) “Scientists Freeze Enzymes and Learn How We React to Drugs”, Rettner, R. MSNBC.com, Nov. 12, 2010. 2) “Elusive Enzyme Species Trapped”, Borman. S., and Kemsley, J. C&E News, 2010, 88, 7. 3) “Scientists Demystify an Enzyme Responsible for Drug and Food Metabolism”, US News and World Reports, Nov. 15, 2010. 4) “Key Player in Detoxification Pathway Isolated After Decades of Searching”, National Science Foundation, Nov. 12, 2010.
5) Oxoiron(IV) in Chloroperoxidase Compound II Is Basic: Implications for P450 Chemistry [link]

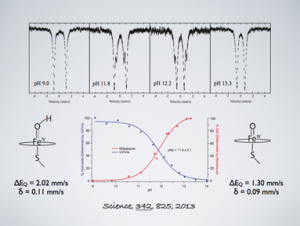
In an effort to understand the role that the axial-thiolate plays in P450 catalysis, we performed x-ray absorption (XAS) experiments on the high-valent forms of chloroperoxidase (CPO). CPO is a thiolate-ligated heme protein that is known cleave activated C-H bonds. The high-valent intermediates in CPO are “relatively” stable and can be prepared in high-yield, making CPO a good model system for studying P450 chemistry. Our XAS experiments yielded important and unexpected results. They revealed a 1.82 Å Fe-O bond in the iron(IV)oxo (or ferryl) form of chloroperoxidase (CPO-II). This distance is too long to be associated with an authentic iron(IV)oxo species. It is indicative of ferryl protonation (pKa > 8). The discovery of the Fe(IV)OH center in CPO-II was notable: no synthetic or mineral iron-hydroxide above the ferric state had been structurally characterized. The real importance of our discovery, however, lies in its connection to P450 chemistry. In the consensus P450 hydroxylation mechanism, compound I (a ferryl-radical species) abstracts hydrogen from substrate to form a protonated ferryl (similar to CPO-II), which rapidly recombines with substrate to yield hydroxylated product. Evidence suggests that the ability of metal-oxo complexes to abstract hydrogen scales with the strength of the O-H bond formed during H-atom abstraction. In heme proteins, the strength of this O-H bond is determined by the one-electron reduction potential of compound I and the pKa of compound II:
D(O-H) = 23.06*E0cmpd-I + 1.37*pKacmpd-II + 57 ± 2 (kcal/mol)
This equation highlights the importance of the ferryl pKa and suggests a role for thiolate-ligation in cytochrome P450, namely to promote hydrogen abstraction at biologically viable compound I reduction potentials. This hypothesis rests upon two important assumptions: 1) that the rebound mechanism is operative in P450 hydroxylations, and 2) that basic ferryls are a general and unique feature of thiolate-ligated hemes.
This work was highlighted in: “Putting the OH where it belongs”, Yarnell, A. C&E News, 2004, 82, 14.

