Research
Protein nanoparticles in cancer immunotherapy
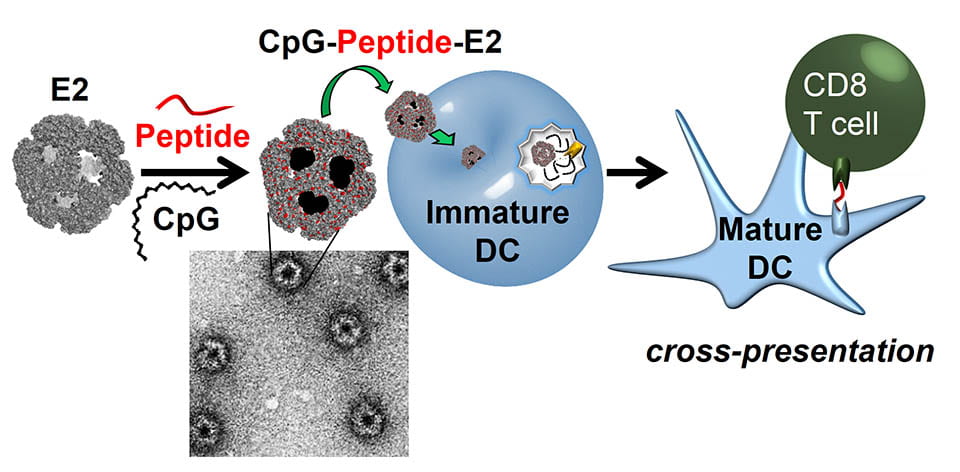
The synthesis of relatively small (<50 nm) and uniform particles with multiple functionalities is often not straightforward using conventional chemical methods, and we are investigating recombinant protein-based nanocontainers as one alternative to traditional polymeric particles. Our platform has been the E2 subunit of pyruvate dehydrogenase, a 25-nm nanoparticle comprised of 60 identical subunits from a thermophilic source. One focus in our lab has been its utility in engineering vaccines.
Cancer immunotherapy aims to recruit the body’s native immune system in the treatment of tumors; however, clinical trials testing cancer antigen vaccines have only demonstrated a weak immunological response. Viruses, in contrast, elicit a strong immune response and are effectively eliminated by the body. Using E2 protein nanoparticles (which are non-viral), we are examining the extent to which an adaptive immune response to tumor-associated antigens can be increased by mimicking the molecular delivery system of viruses. We have shown that formulation of vaccines using E2 to significantly increase antigen-specific anti-tumor responses.
Collagen-mimetic biopolymers for therapeutics and tissue engineering
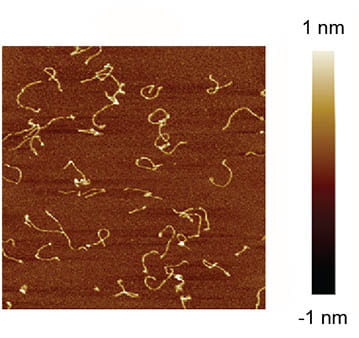
Collagen is the most abundant protein in mammals, and it can dynamically interact with cells. Consequently, researchers have attempted many strategies to fabricate collagen mimics. However, the ability to redesign full-length collagen variants for novel material properties is severely limited by inherent challenges in the gene synthesis and recombinant protein expression of collagen. Our research team has developed a successful strategy to design and synthesize full-length collagen and variants of collagen. We have been examining the ability of these protein polymer mimics to modulate the microenvironment of cells.
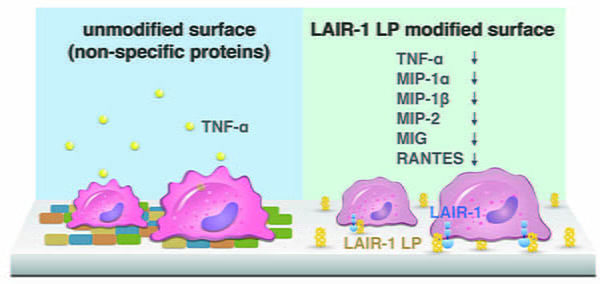
One application of interest is the suppression of inflammatory responses. In nature, immunomodulatory proteins with collagen-like domains can suppress the activation of immune cells through their interaction with the leukocyte-associated Ig-like receptor (LAIR-1). We are investigating the feasibility of decreasing local immune responses, such as inflammation and fibrosis, to implanted surfaces by incorporating LAIR-1 binding sites to materials and their surfaces.
Metabolite-triggered nanomaterials
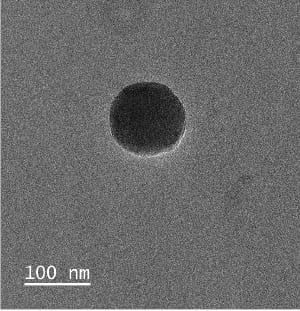
For applications in drug delivery, drug-encapsulating materials are often designed to respond to conditions within the microenvironments of certain tissues or cells. Conventionally, these triggers involve differences in general conditions, such as pH, temperature, or oxidation-reduction conditions. We are developing a new type of material that will respond to signature metabolites of disease. These materials are designed to trigger release of their drug cargo upon encountering specific metabolite concentrations within in vivo microenvironments.
Drug delivery in ophthalmic applications
Drugs for local treatment of eye infections often yield low bioavailability (due to tear production and poor adsorption), resulting in the need for frequent ophthalmic dosing. We are currently investigating controlled-release strategies for ophthalmic formulations that can yield a sustained concentration of drug over longer periods of time.