Publications
[105] Magann, N.; Barnes, G.; Schioldager, R.; Hörmann, F.; Rubén Muñoz; Han, H.; Hoff, D. V.; Vanderwal, C. “Optimized Biomimetic Syntheses of Discorhabdin B and Aleutianamine Drives a Deeper Exploration of Their Anticancer Activities”. ChemRxiv Preprint 2025.
[104] Samkian, A. E.; Nguyen, H. T.; Vanderwal, C. D.; Stoltz, B. M. “On the Unexpected Formation of [2.2.1]- and [2.1.1]Homotriblattanes”. Tetrahedron Lett. 2025, 155591.
[103] Lin, R.; Li, H.; Xiao, Y.; Wang, Z.; Liu, L.; Saalbach, G.; Martins, C.; Furry, M.; Vanderwal, C. D.; Martin, C.; Tatsis, E. C. “Three Cytochrome P450 Enzymes Consecutively Catalyze the Biosynthesis of Furanoclerodane Precursors in Salvia Species.” Plant Communications 2025, 101286.
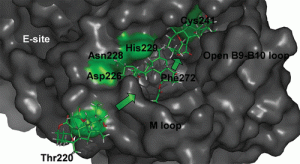
[102] Terrosu, S.; Nurullina, L.; Supantanapong, N.; Pak, B. S. ; Nguyen, S.; Holm, M.; Wu, C.; Lin, M.; Horne, D.; Sachs, M.*; Blanchard, S.*; Yusupov, M.*; Vanderwal, C. D.* “Synthesis of Differentially Halogenated Lissoclimide Analogues to Probe Ribosome E-Site Binding” ACS Chem. Biol, 2025, 20 (4), 858-869
[101] Singh, P.; Choi, J.-Y.; Wang, W.; T. Lam, T.; Lechner, P.; Vanderwal, C. D.; Pou, S.; Nilsen, A.; Ben Mamoun, C. A Fluorescence-Based Assay for Measuring Polyamine Biosynthesis Aminopropyl Transferase–Mediated Catalysis. J. Bio. Chem. 2024, 300, 107832.
[100] Barnes, G. L.; Magann, N. L.; Perrotta, D.; Hörmann, F. M.; Fernandez, S.; Vydyam, P.; Choi, J.-Y.; Prudhomme, J.; Neal, A.; Le Roch, K. G.; Ben Mamoun, C.; Vanderwal, C. D. A “Divergent Synthesis of Numerous Pyrroloiminoquinone Alkaloids Identifies Promising Antiprotozoal Agents.” J. Am. Chem. Soc. 2024, 146, 29883-29894.

[99] Chahine, Z.; Abel, S.; Hollin, T.; Barnes, G. L.; Chung, J. H.; Daub, M. E.; Renard, I.; Choi, J. Y.; Vydyam, P.; Pal, A.; Alba-Argomaniz, M.; Banks, C. A. S.; Kirkwood, J.; Saraf, A.; Camino, I.; Castaneda, P; Cuevas, M. C.; De Mercado-Arnanz, J.; Fernandez-Alvaro, E.; Garcia-Perez, A.; Ibarz, N.; Viera-Morilla, S.; Prudhomme, J.; Joyner, C. J.; Bei, A. K.; Florens, L.; Ben Mamoun, C.*; Vanderwal, C. D.*; Le Roch, K. G.* “A Kalihinol Analogue Disrupts Apicoplast Function and Vesicular Trafficking in P. falciparum Malaria” Science 2024 , 385 (6716), eadm7966.

[98] Park, B. J.; Vanderwal, C. D. “Lessons Learned: Asphyxiation Hazard Associated with Dry Ice” ACS Chem. Health Saf. 2023, 30, 120–123.
[97] Niman, S. W.; Buono, R.; Fruman, D. A.; Vanderwal, C. D. “Synthesis of a Complex Brasilicardin Analogue Utilizing a Cobalt-Catalyzed MHAT-Induced Radical Bicyclization Reaction” Org. Lett. 2023, 25, 3451–3455.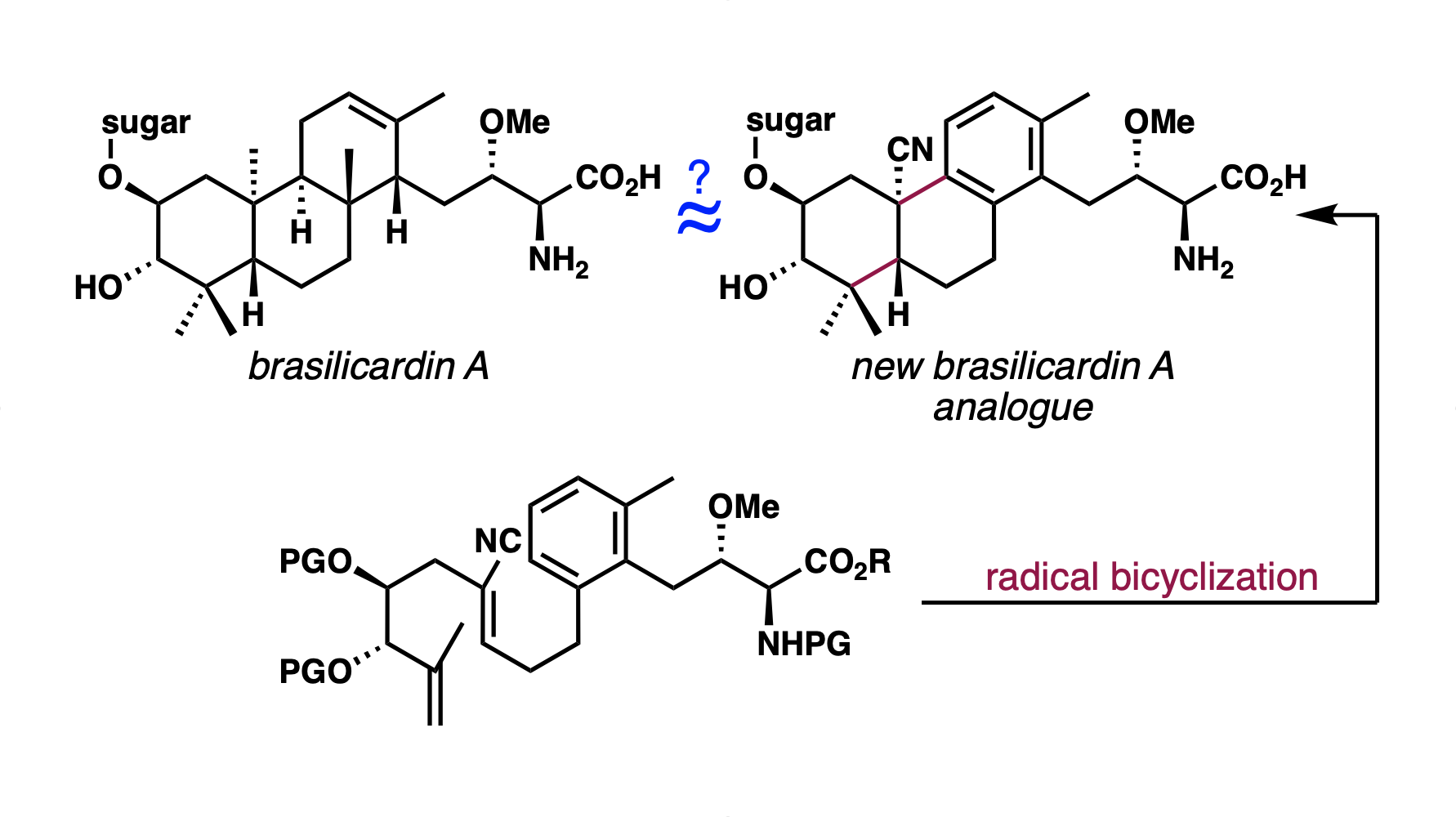
[96] Kozlowski, R. A.; Nguyen, H. T.; Lehman, M. E.; Vanderwal, C. D. “Stereocontrolled Access to Quaternary Centers by Birch Reduction/Alkylation of Chiral Esters of Salicylic Acids” J. Org. Chem. 2023, 88, 6232–6236.
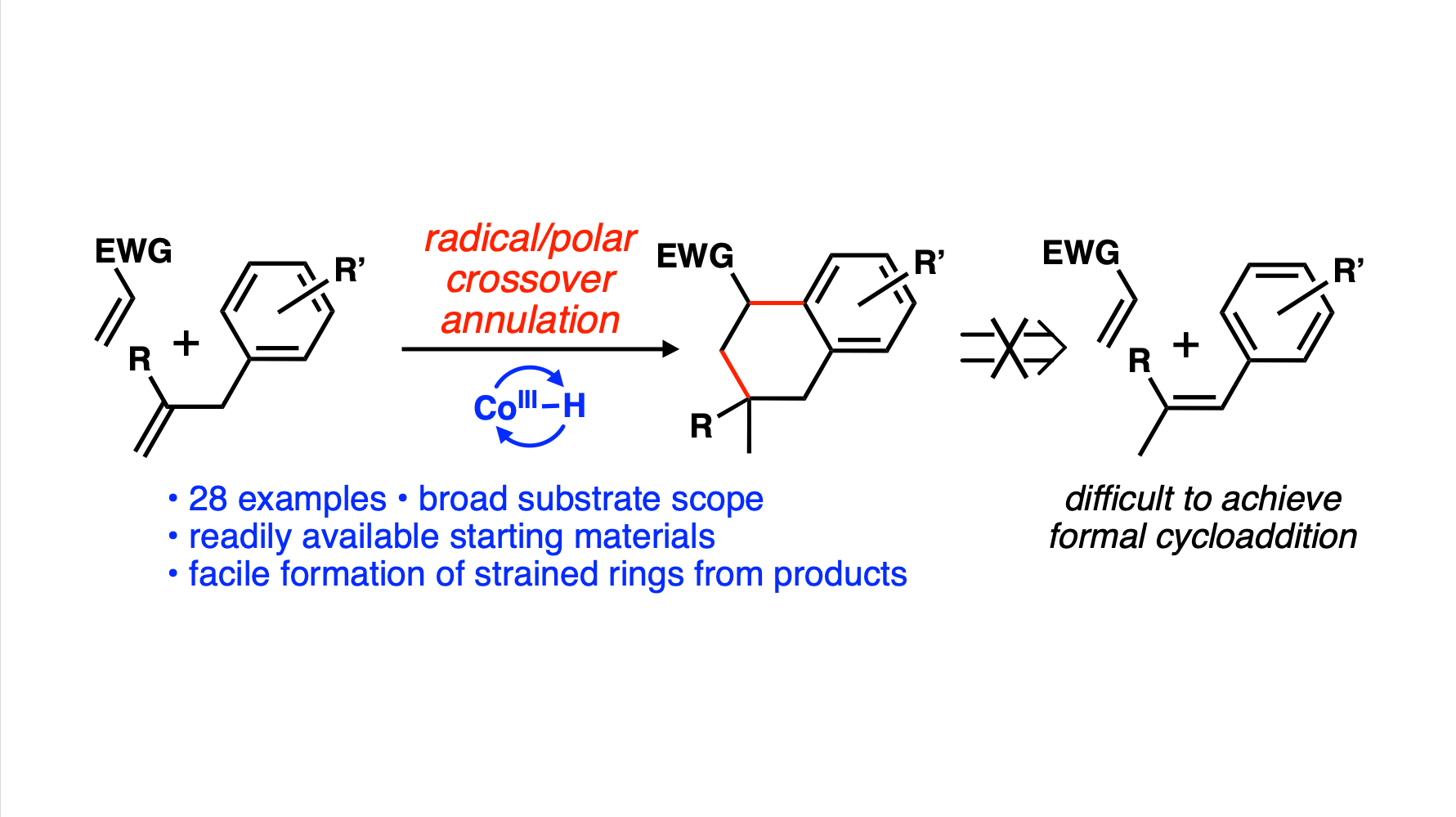
[95] Johnson, L. K.; Barnes, G. L.; Fernandez, S. A.; Vanderwal, C. D. “Hydrogen-Atom-Transfer-Initiated Radical/Polar Crossover Annulation Cascade for Expedient Access to Complex Tetralins” Angew. Chem. Int. Ed. 2023, e202303228
[94] Chung, J.; Capani Jr., J. S.; Göhl, M.; Roosen, P. C.; Vanderwal, C. D. “Enantioselective Syntheses of Wickerols A and B“ J. Am. Chem. Soc. 2023, 145, 6486–6497.
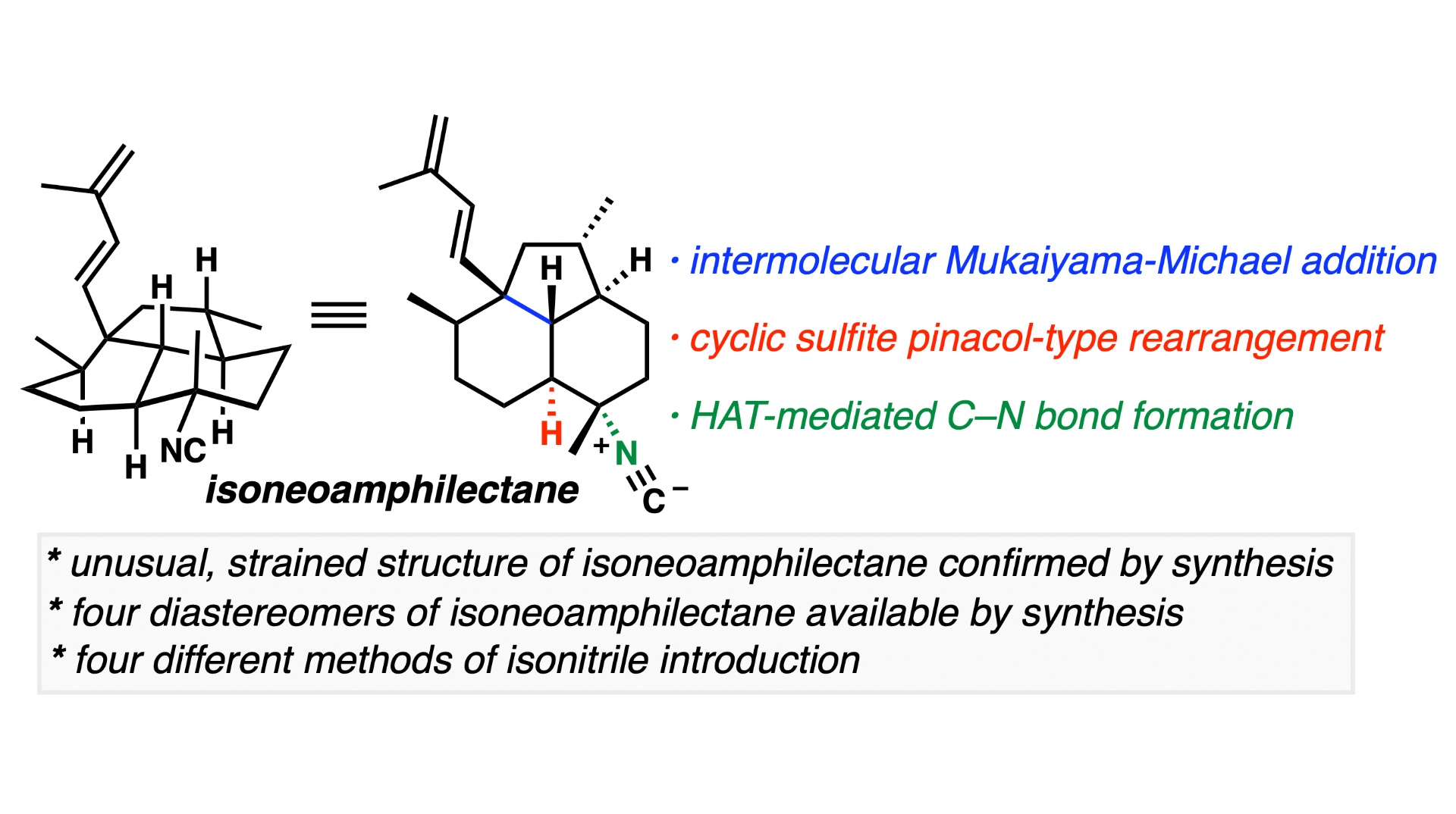
[93] Dwulet, N. C.; Chahine, Z.; Le Roch, K. G.; Vanderwal, C. D. “An Enantiospecific Synthesis of Isoneoamphilectane Confirms Its Strained Tricyclic Structure“ J. Am. Chem. Soc. 2023, 145, 3716–3726.
[92] Barnes, G. L.; Hong, A. Y.; Vanderwal, C. D. “A Synthesis of Alstonlarsine A via Alstolucines B and F Demonstrates the Chemical Feasibility of a Proposed Biogenesis” Angew. Chem. Int. Ed. 2022, e202215098
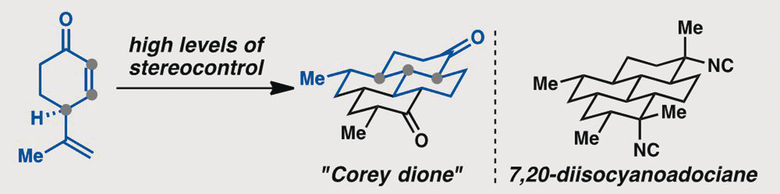
[91] Roosen, P. C.; Karns, A. S.; Ellis, B. D.; Vanderwal, C. D. “Evolution of a Short and Stereocontrolled Synthesis of (+)-7,20-Diisocyanoadociane” J. Org. Chem. 2022, 87, 1398–1420.
[90] Dwulet, N. C.; Romella, V.; Vanderwal, C. D. “Soft Enolization of 3-Substituted Cycloalkanones Exhibits Significantly Improved Regiocontrol vs Hard Enolization Conditions” Org. Lett. 2021, 23, 9616–9619.
[89] Johnson, L. K.; Niman, S. W.; Vrubliauskas, D.; Vanderwal, C. D. “Sterecontrolled Synthesis and Structural Revision of Plebeianiol A” Org. Lett. 2021, 23, 9569–9573.
[88] Vrubliauskas, D.; Groß, B.; Vanderwal, C. D. “Stereocontrolled Radical Bicyclizations of Oxygenated Precursors Enables Short Syntheses of Oxidized Abietane Diterpenoids” J. Am. Chem. Soc. 2021, 143, 2944–2952.

[87] Pak, B. S.; Supantanapong, N.; Vanderwal, C. D. “The Recurring Roles of Chlorine in Synthetic and Biological Studies of the Lissoclimides” Acc. Chem. Res. 2021, 54, 1131–1142.
[86] Vanderwal, C. D.; Romo, D. “Editorial: Synthetic Strategies for Mining the Information-Rich Content of Natural Products for Biology and Medicine” Nat. Prod. Rep. 2020, 37, 1393–1394.
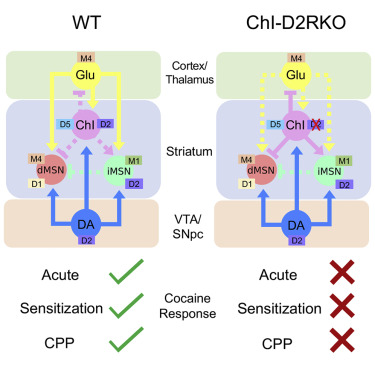
[85] Lewis, R. G.; Serra, M.; Radl, D.; Gori, M.;Tran, C.; Michalak, S. E.; Vanderwal, C. D.; Borrelli, E. “Dopaminergic Control of Striatal Cholinergic Interneurons Underlies Cocaine-Induced Psychostimulation” Cell Reports 2020, 31, 107527–107535.
[84] Ramella, V.; Roosen, P. C.; Vanderwal, C. D. “Concise Formal Synthesis of the Pseudopterosins via Anionic OxyCope/Transannular Michael Addition Cascade” Org. Lett. 2020, 22, 2883–2886.
[83] Vrubliauskas, D.; Vanderwal, C. D. “Cobalt-Catalyzed Hydrogen Atom Transfer Induces Bicyclizations that Tolerate Electron-rich and Electron–deficient Intermediate Alkenes” Angew. Chem. Int. Ed. 2020, 59, 6115–6121.
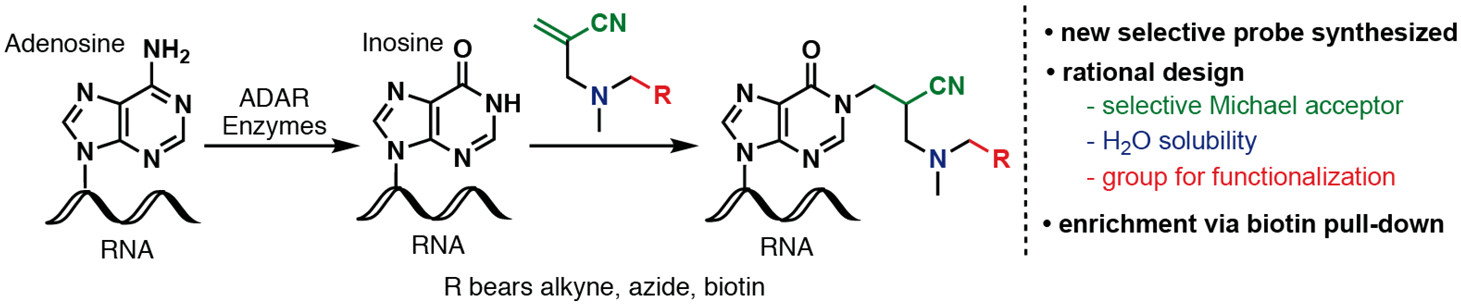
[82] Li, Y.; Göhl, M.; Ke, K.; Vanderwal, C. D.; Spitale, R. C. “Identification of Adenosine-to-Inosine RNA Editing with Acrylonitrile Reagents” Org. Lett. 2019, 21, 7948–7951.
[81] Gaffary, M. E.; Hafner, S.; Lang, S. J.; Jin, L.; Sabry, O. M.; Vogel, C. V.; Vanderwal, C. D.; Syrovets, T.; Simmet, T. “A Novel Polyhalogenated Monoterpene Induces Cell Cycle Arrest and Apoptosis in Breast Cancer Cells” Mar. Drugs 2019, 17, 437–452.
[80] Karns, A. S.; Ellis, B. D.; Roosen, P. C.; Chahine, Z.; Le Roch, K. G.; Vanderwal, C. D. “Concise Synthesis of the Antiplasmodial Isocyanoterpene 7,20-Diisocyanoadociane” Angew. Chem. Int. Ed. 2019, 58, 1–5.
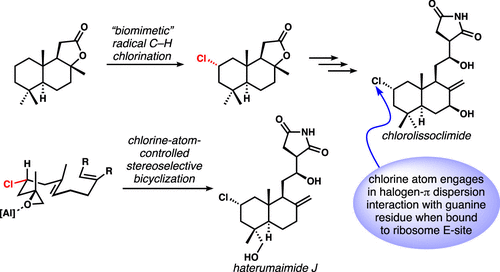
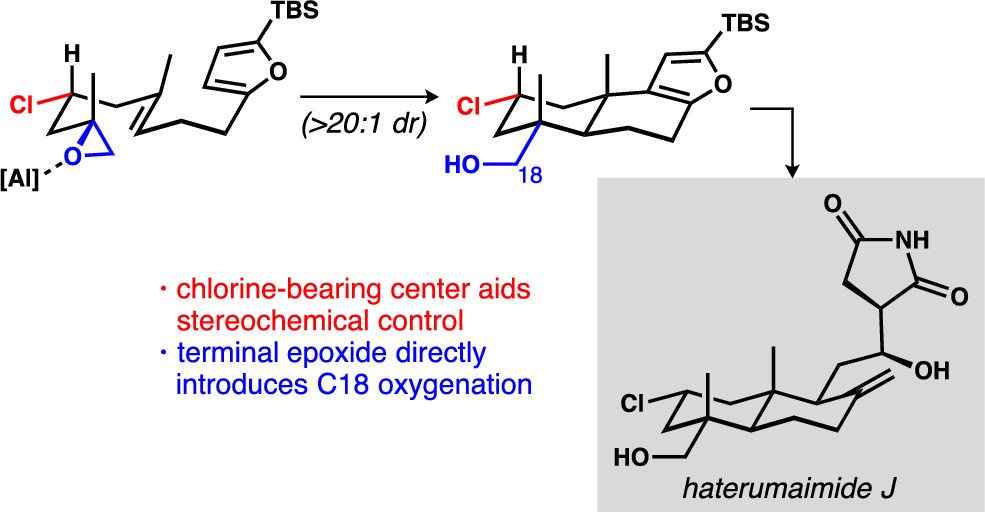
[79] Michalak, S. E.; Nam, S.; Kwon, D. M.; Horne, D. A; Vanderwal, C. D. “A Chlorine-Atom-Controlled Terminal-Epoxide-Initiated Bicyclization Cascade Enables a Synthesis of the Potent Cytotoxins Haterumaimides J and K” J. Am. Chem. Soc. 2019, 141, 9202–9206.
[78] Pelligrino, S.; Meyer, M.; Könst, Z. A.; Holm, M.; Voora, V. K.; Kashinskaya, D.; Zanette, C.; Mobley, D. L.; Yusupova, G.; Vanderwal, C. D.; Blanchard, S. C.; Yusupov, M. “Understanding the Role of Intermolecular Interactions between Lissoclimides and the Eukaryotic Ribosome” Nucleic Acids Research 2019, 47, 3223–3232.
[77] Balaguer, F. A.; Mühlethaler, T.; Estévez-Gallego, J.; Calvo, E.; Giménez-Abián, J. F.; Risinger, A. L.; Sorensen, E. J.; Vanderwal, C.D.; Altmann, K. H.; Mooberry, S. L.; Steinmetz, M. O.; Oliva, M. Á.; Prota, A. E.; Díaz, J. F. “Crystal Structure of the Cyclostreptin-Tubulin Adduct: Implications for Tubulin Activation by Taxane-Site Ligands” Int. J. Mol. Sci. 2019, 20, 1392–1399.
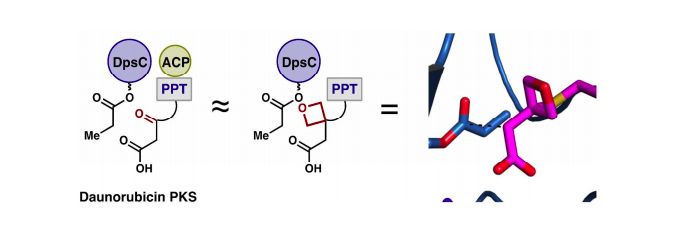
[76] Ellis, B. D.; Milligan, J. C.; White, A. R.; Duong, V.; Altman, P. X.; Mohammed, L.; Crump, M. P.; Crosby, J.; Luo, R.; Vanderwal, C. D.; Tsai, S. C. “An Oxetane-based Polyketide Surrogate to Probe Substrate Binding in a Polyketide Synthase” J. Am. Chem. Soc. 2018, 140, 4961–4964.
[75] White, A. M.; Dao, K.; Vrubliauskas, D.; Konst, Z. A.; Pierens, G. K.; Mándi, A.; Andrews, K. T.; Skinner-Adams, T. S.; Clarke, M. E.; Narbutas, P. T.; Sim, D. C.-M.; Cheney, K. L.; Kurtán, T.; Garson, M. J.; Vanderwal. C. D “Catalyst-Controlled Stereoselective Synthesis Secures the Structure of the Antimalarial Isocyanoterpene Pustulosaisonitrile-1” J. Org. Chem. 2017, 82, 13313–13323.

[74] Ellis, B. D.; Vanderwal, C. D. “Hughes and Gleason’s Virosaine A – Appreciating Art in Synthesis” Angew. Chem. Int. Ed. 2017, 56, 13940–13942.
[73] Herzon, S. B.; Vanderwal, C. D. “Introduction: Natural Products Synthesis” Chem. Rev. 2017, 117, 11649–11650.
[72] Mai, D. N.; Uchenik, D.; Vanderwal, C. D. “Efforts Toward a Synthesis of Crotogoudin and Crotobarin” Synlett 2017, 28, 1758–1762.

[71] White, A. R.; Kozlowski, R. A.; Tsai, S.-C.; Vanderwal, C. D. “A Direct Synthesis of Highly Substituted π-Rich Aromatic Heterocycles from Oxetanes” Angew. Chem. Int. Ed. 2017, 56, 10525–10529.
[70] Könst, Z. A.; Szklarski, A. R.; Michalak, S. E.; Pellegrino, S.; Meyer, M.; Zanette, C.; Cencil, R.; Nam, S.; Voora, V.; Horne, D. A.; Pelletier, J.; Mobley, D. L.; Yusupov, M.*; Vanderwal, C. D. “Synthesis Facilitates an Understanding of the Structural Basis for Translation Inhibition by the Lissoclimides” Nature Chem, 2017, 9, 1140–1149.

[69] Atwood, B. A.; Vanderwal, C. D. “Recent Advances in Alkene Metathesis for Natural Product Synthesis – Striking Achievements Resulting from Increased Sophistication in Catalyst Design and Synthesis Strategy” Aldrichimica Acta, 2017, 50, 17–27.
[68] Daub, M. E.; Roosen, P. C.; Vanderwal, C. D. “General Approaches to Structurally Diverse Isocyanoditerpenes” J. Org. Chem. 2017, 82, 4533–4541.
[67] Daub, M. E.; Prudhomme, J.; Ben Mamoun, C.; Le Roch, K. G.; Vanderwal, C. D. “Antimalarial Properties of Simplified Kalihinol Analogues” ACS Med. Chem. Lett. 2017, 8, 355–360.

[66] Schwarzwalder, G. M.; Vanderwal, C. D. “Strategies for the Synthesis of the Halenaquinol and Xestoquinol Families of Natural Products” Eur. J. Org. Chem. 2017, 12, 1567–1577
[65] Di, K.; Lomeli, N.; Wood, S. D.; Vanderwal, C. D.; Bota, D. A. “Mitochondrial Lon is Over-Expressed in High-Grade Gliomas, and Mediates Hypoxic Adaptation: Potential Role of Lon as a Therapeutic Target in Glioma” Oncotarget 2016, 7, 77457–77467.
[64] Hong, A. Y.; Vanderwal, C. D. “A Sequential Cycloaddition Strategy for the Synthesis of Alsmaphorazine B Traces a Path Through a Family of Alstonia Alkaloids” Tetrahedron 2016, 73, 4160–4171.

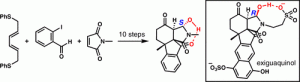
[63] Schwarzwalder, G. M.; Scott, D. R.; Vanderwal, C. D. “A Synthesis of Exiguaquinol Dessulfate” Chem. Eur. J. 2016, 22, 17953–17957
 [62] Roosen, P. C.; Vanderwal, C. D. “A Formal Enantiospecific Synthesis of 7,20-Diisocyanoadociane” Angew. Chem. Int. Ed. 2016, 55, 7180–7183.
[62] Roosen, P. C.; Vanderwal, C. D. “A Formal Enantiospecific Synthesis of 7,20-Diisocyanoadociane” Angew. Chem. Int. Ed. 2016, 55, 7180–7183.
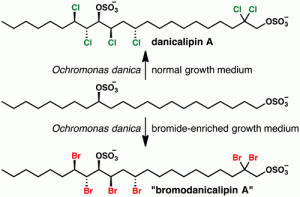
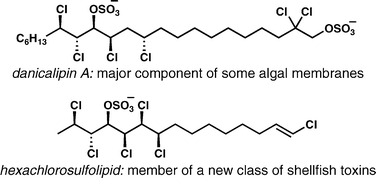
[61] White, A. R.; Duggan, B. M.; Tsai, S.-C.; Vanderwal, C. D. “The Alga Ochromonas danica Produces Bromosulfolipids” Org. Lett. 2016, 18, 1124–1127.
[60] Horn, E. J.; Silverston, J. S.; Vanderwal, C. D. “A Failed Late-Stage Epimerization Thwarts an Approach to Ineleganolide” J. Org. Chem. 2016, 81, 1819–1838.
[59] Chung, W.-j.; Vanderwal, C. D. “Stereocontrolled Halogenation in Natural Product Synthesis” Angew. Chem. Int. Ed. 2016, 55, 4396–4434.
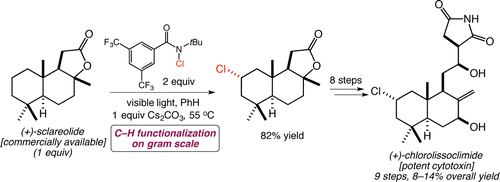
[58] Quinn, R. K.; Konst, Z. A.; Michalak, S. E.; Schmidt, Y.; Szklarski, A. R.; Flores, A. R.; Nam, S.; Horne, D. A.; Vanderwal, C. D.; Alexanian, E. J. “Site-Selective Aliphatic C-H Chlorination using N-Chloroamides Enables a Synthesis of Chlorolissoclimide” J. Am. Chem. Soc. 2016, 138, 696–702.
[57] Hong, A. Y.; Vanderwal, C. D. “A Synthesis of Alsmaphorazine B Demonstrates the Chemical Feasibility of a New Biogenetic Hypothesis” J. Am. Chem. Soc. 2015, 137, 7306–7309.
[56] Pham, H. V.; Karns, A. S.; Vanderwal, C. D.; Houk, K. N. “Computational and Experimental Investigations of the Formal Dyotropic Rearrangements of Himbert Arene/Allene Cycloadducts” J. Am. Chem. Soc. 2015, 137, 6956–6964.
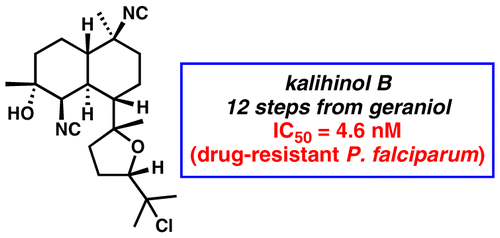
[55] Daub, M. E.; Prudhomme, J.; Le Roch, K.; Vanderwal, C. D. “Synthesis and Potent Antimalarial Activity of Kalihinol B” J. Am. Chem. Soc. 2015, 137, 4912–4915.
[54] Romonovsky, D. E.; Nguyen, L. Q.; Shemesh, D.; Nguyen, T. B.; Epstein, S. A.; Martin, D. B. C.; Vanderwal, C. D.; Gerber, R. B.; Nizkorodov, S. A. “Absorption Spectra and Aqueous Photochemistry of b-Hydroxyalkyl Nitrates of Atmospheric Interest” Mol. Phys. 2015, 113, 2179–2190. (Special Issue in Honour of John Maier).
[53] Atwood, B. R; Vanderwal, C. D. “News & Views: Catalytic Control of Chlorination” Nature Chemistry 2015, 7, 99–100.
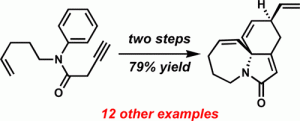
[52] Lam, J. K.; Joseph, S. B.; Vanderwal, C. D. “A Zincke Aldehyde Approach to Gelsemine” Tetrahedron Lett. 2015, 56, 3165–3168. (Special Issue to Honor the Memory of Professor Harry Wasserman).

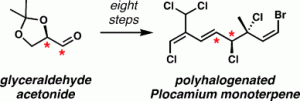
[51] Vogel, C. V.; Pietraszkiewicz, H.; Sabry, O. M.; Gerwick, W. H.; Valeriote, F. A.; Vanderwal, C. D. “Enantioselective, Divergent Syntheses of Several Polyhalogenated Plocamium Monoterpenes and Evaluation of their Selectivity for Solid Tumors” Angew. Chem. Int. Ed. 2014, 53, 12205–12209.
[50] Roosen, P. C.; Vanderwal, C. D. “Investigations into an Anionic Oxy-Cope/Transannular Conjugate Addition Approach to 7,20-Diisocyanoadociane” Org. Lett. 2014, 16, 4368–4371.
[49] Vanderwal, C. D. “Allylsilane RCM/Electrophilic Desilylation as a Means to Access Rings with Exocyclic Alkenes” Handbook of Metathesis, Grubbs, R. H.; O’Leary, D., Eds.; Wiley-VCH: Weinheim, 2015; Vol. 2, pp 563-573.
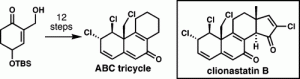
[48] Tartakoff, S. S.; Vanderwal, C. D. “A Synthesis of the ABC Tricyclic Core of the Clionastatins Serves to Corroborate their Proposed Structures” Org. Lett. 2014, 16, 1458–1461.
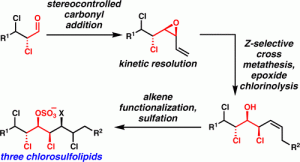
[47] Chung, W.-j.; Carlson, J. S.; Vanderwal, C. D. “A General Approach to the Synthesis of the Chlorosulfolipids Danicalipin A, Mytilipin A, and Malhamensilipin A in Enantioenriched Form” J. Org. Chem. 2014, 79, 2226–2241.
[46] Chung, W.-j.; Vanderwal, C. D. “Approaches to the Chemical Synthesis of the Chlorosulfolipids” Acc. Chem. Res. 2014, 47, 718–728.
[45] Schwarzwalder, G. M.; Steinhardt, S. E.; Pham, H. V.; Houk, K. N.; Vanderwal, C. D. “A Synthesis of the Tetracyclic Core of Exiguaquinol” Org. Lett. 2013, 15, 6014–6017.
[44] Lam, J. K.; Pham, H. V.; Houk, K. N.; Vanderwal, C. D. “Computation and Experiment Reveal that the Ring-Rearrangement Metathesis of Himbert Cycloadducts Can Be Subject to Kinetic or Thermodynamic Control” J. Am. Chem. Soc. 2013, 135, 17585–17594.
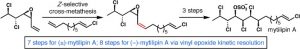
[43] Chung, W.-j.; Carlson, J. S.; Bedke, D. K.; Vanderwal, C. D. “A Synthesis of the Chlorosulfolipid Mytilipin A via a Longest Linear Sequence of Seven Steps” Angew. Chem. Int. Ed. 2013, 52, 10052–10055.
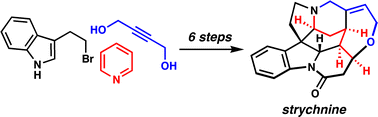
[42] Martin, D. B. C.; Vanderwal, C. D. “A Short Synthesis of Strychnine from Pyridine” In Total Synthesis of Natural Products: At the Frontier of Organic Chemistry; Li, J. J. and Corey, E. J. Eds.; Springer: Heidelberg; 2013; pp 67–102.
[41] Schmidt, Y.; Lam, J. K.; Pham, H. V.; Houk, K. N.; Vanderwal, C. D. “Studies on the Himbert Intramolecular Arene/Allene Diels–Alder Cycloaddition. Mechanistic Studies and Expansion of Scope to All-Carbon Tethers.” J. Am. Chem. Soc. 2013, 135, 7339–7348.
[40] Lam, J. K.; Schmidt, Y.; Vanderwal, C. D. “Complex Polycyclic Scaffolds by Metathesis Rearrangement of Himbert Arene/Allene Cycloadducts” Org. Lett. 2012, 14, 5566–5569.
[39] Michels, T. D.; Dowling, M. S.; Vanderwal, C. D. “A Synthesis of Echinopine B” Angew. Chem. Int. Ed. 2012, 51, 7572–7576.
[38] Pham, H. V.; Martin, D. B. C.; Vanderwal, C. D.; Houk, K. N. “The Intramolecular Diels–Alder Reaction of Tryptamine-Derived Zincke Aldehydes is a Stepwise Process” Chem. Sci. 2012, 3, 1650–1655.
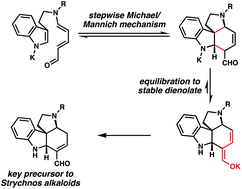
[37] Martin, D. B. C.; Nguyen, L. Q.; Vanderwal, C. D. “Syntheses of Strychnine, Norfluorocurarine, Dehydrodesacetylretuline, and Valparicine Enabled by Intramolecular Cycloadditions of Zincke Aldehydes” J. Org. Chem. 2012, 77, 17–46.
[36] Calvo, E.; Barasoain, I.; Matesanz, R.; Pera, B.; Camafeita, E.; Pineda, O.; Hamel, E.; Vanderwal, C. D.; Andreu, J. M.; López, J. A.; Díaz, J. F. “Cyclostreptin Derivatives Specifically Target Cellular Tubulin and Further Map the Paclitaxel Site” Biochemistry 2012, 51, 329–341.
[35] Vanderwal, C. D. “Reactivity and Synthesis Inspired by the Zincke Ring-Opening of Pyridines” J. Org. Chem. (Perspective) 2011, 76, 9555–9567.
[34] Paton, R. S.; Steinhardt, S. E.; Vanderwal, C. D.; Houk, K. N. “Unraveling the Mechanism of Cascade Reactions of Zincke Aldehydes” J. Am. Chem. Soc. 2011, 133, 3895–3905.
[33] Martin, D. B. C.; Vanderwal, C. D. “A Synthesis of Strychnine via a Longest Linear Sequence of Six Steps” Chem. Sci. 2011, 2, 649–651.
[32] Bedke, D. K.; Vanderwal, C. D. “Chlorosulfolipids: Structure, Synthesis, and Biological Relevance” Nat. Prod. Rep. 2011, 28, 15–25.
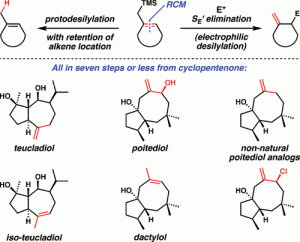
[31] Dowling, M. S.; Vanderwal, C. D. “Ring-Closing Metathesis of Allylsilanes as a Flexible Strategy Toward Cyclic Terpenes. Short Syntheses of Teucladiol, Isoteucladiol, Poitediol and Dactylol, and an Attempted Synthesis of Caryophyllene” J. Org. Chem. 2010, 75, 6908–6922.
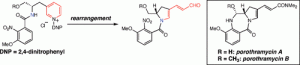
[30] Michels, T. D.; Kier, M. J.; Kearney, A. M. Vanderwal, C. D. “Concise Formal Syntheses of Porothramycins A and B via Zincke Pyridinium Ring-Opening/Ring-Closing Cascade” Org. Lett. 2010, 12, 3093–3095.
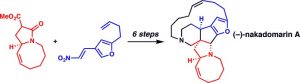
[29] Martin, D. B. C.; Vanderwal, C. D. “Highlight: Concise Synthesis of (–)-Nakadomarin A” Angew. Chem. Int. Ed. 2010, 49, 2830–2832.
[28] Bedke, D. K.; Shibuya, G. M.; Pereira, A. R.; Gerwick, W. H.; Vanderwal, C. D. “A Concise Enantioselective Synthesis of the Chlorosulfolipid Malhamensilipin A” J. Am. Chem. Soc. 2010, 132, 2542–2543.

[27] Pereira, A. R.; Byrum, T.; Shibuya, G. M.; Vanderwal, C. D.; Gerwick, W. H. “Structure Revision and Absolute Configuration of Malhamensilipin A from the Freshwater ChrysophytePoterioochromonas malhamensis.” J. Nat. Prod. 2010, 73, 279–283.

[26] Steinhardt, S. E.; Vanderwal, C. D. “News and Views: Without a Trace” Nature Chemistry 2010, 2, 254–256.
[25] Bedke, D. K.; Vanderwal, C. D. “Tetraethylammonium trichloride” eEROS 2010.
[24] Dowling, M. S.; Vanderwal, C. D. “Ring-Closing Metathesis of Allylsilanes/Electrophilic Desilylation to Prepare exo-Methylidenecycloalkanes. Short Syntheses of Teucladiol and Poitediol” J. Am. Chem. Soc. 2009, 131, 15090–15091.

[23] Bedke, D. K.; Shibuya, G. M.; Pereira, A.; Gerwick, W. H.; Haines, T. H.; Vanderwal, C. D.“Relative Stereochemistry Determination and Synthesis of the Major Chlorosulfolipid from Ochromonas danica” J. Am. Chem. Soc. 2009, 131, 7570–7572.

[22] Steinhardt, S. E.; Vanderwal, C. D. “Complex Polycyclic Lactams from Pericyclic Cascade Reactions of Zincke Aldehydes” J. Am. Chem. Soc. 2009, 131, 7546–7547.

[21] Martin, D. B. C.; Vanderwal, C. D. “Efficient Access to the Core of the Strychnos, Aspidosperma and Iboga Alkaloids. A Short Synthesis of Norfluorocurarine.” J. Am. Chem. Soc. 2009, 131, 3472–3473.

[20] Kanady, J. S.; Nguyen, J. D.; Ziller, J. W.; Vanderwal, C. D. “Synthesis and Characterization of All Four Diastereomers of 3,4-Dichloro-2-pentanol, Motifs Relevant to the Chlorosulfolipids” J. Org. Chem. 2009, 74, 2175–2178.

[19] Bedke, D. K.; Vanderwal, C. D. “Chlorine Lends a Helping Hand” News and Views Article in Nature, 2009, 457, 548–549.
[18] Michels, T. D.; Rhee, J. U.; Vanderwal, C. D. “Synthesis of δ-Tributylstannyl-α,β,γ,δ-unsaturated Aldehydes from Pyridines” Org. Lett. 2008, 10, 4787–4790.

[17] Shibuya, G. M.; Kanady, J. S.; Vanderwal, C. D. “Stereoselective Dichlorination of Allylic Alcohol Derivatives to Access Key Stereochemical Arrays of the Chlorosulfolipids” J. Am. Chem. Soc. 2008, 130, 12514–12518.

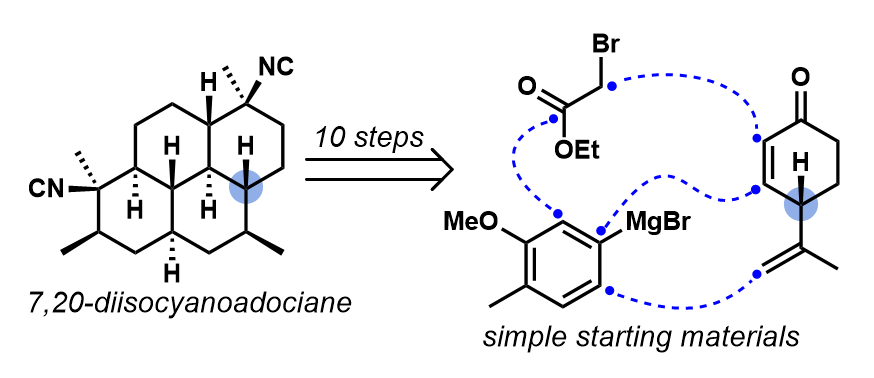
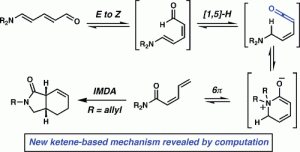
[16] Steinhardt, S. E.; Silverston, J. S.; Vanderwal, C. D. “Stereocontrolled Synthesis of Z-Dienes via an Unexpected Pericyclic Cascade Rearrangement of 5-Amino-2,4-pentadienals” J. Am. Chem. Soc. 2008, 130, 7560–7561.

[15] Bai, R.; Vanderwal, C. D.; Díaz, J. F.; Hamel, E. “The Interaction of a Cyclostreptin Analogue with the Microtubule: The Covalent Reaction Rapidly Follows Binding” J. Nat. Prod. 2008, 71, 370–374.

[14] Soenen, D. R.; Vanderwal, C. D. “Product Class 37.1 Alkyl Ethers; 37.1.3 Synthesis of Ethers by Addition to Alkenes” In Science of Synthesis; Volume 37; Forsyth, C. J., Volume Editor; Georg Thieme Verlag: Stuttgart, 2008; p. 99.
[13] Buey, R. M.; Calvo, E.; Barasoain, I.; Pineda, O.; Edler, M. C.; Matesanz, R.; Cerezo, G.; Vanderwal, C. D.; Day, B. W.; Sorensen, E. J.; López, J. A.; Andreu, J. M.; Hamel, E.; Diaz, J. F.“Cyclostreptin Binds Covalently to Microtubule Pores and Lumenal Taxoid Binding Sites” Nat. Chem. Bio. 2007, 3, 117–125.
[12] Kearney, A. M.; Vanderwal, C. D. “Synthesis of Nitrogen Heterocycles by the Ring Opening Reaction of Pyridinium Salts” Angew. Chem. Int. Ed. 2006, 45, 7803.

[11] Vanderwal, C. D.; Jacobsen, E. N. “Product Subclass 11: Alk-2-enoic Acids” In Science of Synthesis; Volume 20a; Panek, J. S., Volume Editor; Georg Thieme Verlag: Stuttgart, 2006; p 1305.
[10] Vanderwal, C. D.; Jacobsen, E. N. “Product Subclass 14: Alk-2-enoic Acid Esters” In Science of Synthesis; Volume 20b; Panek, J. S., Volume Editor; Georg Thieme Verlag: Stuttgart, 2006; p 551.
[9] Edler, M. C.; Buey, R. M.; Gussio, R.; Marcus, A. I.; Vanderwal, C. D.; Sorensen, E. J.; Diaz, J. F.; Giannakakou, P.; Hamel, E. “Cyclostreptin (FR182877), an Antitumor Tubulin-Polymerizing Agent Deficient in Enhancing Tubulin Assembly Despite Its High Affinity for the Taxoid Site” Biochemistry 2005, 44, 11525.

[8] Vanderwal, C. D.; Jacobsen, E. N. “Enantioselective Formal Hydration of α,β-Unsaturated Imides by Al-Catalyzed Conjugate Addition of Oxime Nucleophiles” J. Am. Chem. Soc. 2004, 126, 14724.

[7] Vanderwal, C. D.; Sorensen, E. J. “A Remarkable Architectural Self-Construction Process is Discovered in a Synthesis of FR182877” In Strategies and Tactics in Organic Synthesis, Volume 5; Harmata, M. Ed.; Elsevier Academic Press: Oxford, 2004; Vol. 5, pp 1-50.
[6] Adam, G. C.; Vanderwal, C. D.; Sorensen, E. J.; Cravatt, B. F. “(–)-FR182877 is a Potent and Selective Inhibitor of Carboxylesterase-1.” Angew. Chem. Int. Ed. 2003, 42, 5480. (Shared first authorship with G. C. Adam).
[5] Vanderwal, C. D.; Vosburg, D. A.; Weiler, S.; Sorensen, E. J. “An Enantioselective Synthesis of FR182877 Provides a Chemical Rationalization of Its Structure and Affords Multi-gram Quantities of Its Direct Precursor” J. Am. Chem. Soc. 2003, 125, 5393.

[4] Vosburg, D. A.; Vanderwal, C. D.; Sorensen, E. J. “A Synthesis of (+)-FR182877 Featuring Tandem Transannular Diels-Alder Reactions Inspired by a Postulated Biogenesis” J. Am. Chem. Soc. 2002, 124, 4552.

[3] Vanderwal, C. D.; Vosburg, D. A.; Sorensen, E. J. “Intramolecular Allenolate Acylations in Studies toward a Synthesis of FR182877” Org. Lett. 2001, 3, 4307.

[2] Vanderwal, C. D.; Vosburg, D. A.; Weiler, S.; Sorensen, E. J. “Postulated Biogenesis of WS9885B and Progress toward an Enantioselective Synthesis” Org. Lett. 1999, 1, 645.

[1] Friesen, R. W.; Vanderwal, C. “Total Synthesis of (±)-Dihydrokawain-5-ol. Regioselective Monoprotection of Vicinal Syn-Diols Derived from the Iodocyclofunctionalization of α-Allenic Alcohols” J. Org. Chem. 1996, 61, 9103.