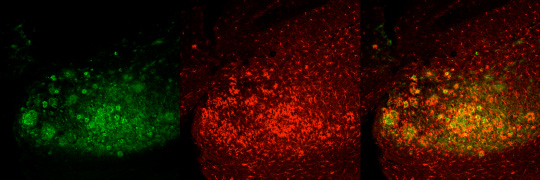
Microglia are heavily implicated in almost all neurodegenerative diseases, as well as traumatic brain injuries and strokes. For example, microglia are heavily implicated in the progression of AD. They are thought to have roles in both the initial development of AD pathologies (i.e. plaques and tangles), as well as the response to these pathologies, whereby they mount a chronic neuroinflammatory response that is never resolved and likely contributes towards the synaptic and neuronal loss, as well as the cognitive decline. The AD brain is characterized by the presence of activated microglia surrounding dense core plaques (as shown below). These microglia are attempting to phagocytose and clear Abeta from the brain, but are unable to do so resulting in sustained activation and the secretion of many pro-inflammatory cytokines throughout the course of the disease. Activated microglia are detectable via PET imaging with ligands directed against TPSO, and imaging in AD patients shows good correlations with cognitive decline, unlike plaque load.
Additionally, aberrant microglia are implicated in the response to brain injuries, and may impede functional recovery following injury. We have discovered that microglia in the healthy adult brain are fully dependent upon CSF1R signaling for their survival, and that chronic elimination of microglia does not lead to any overt deficits in behaviour, or learning and memory. As such we are now turning to explore how CSF1R inhibitors can be used in various disease states. We have 2 important questions to address:
1) Are disease and injury-activated microglia still dependent upon CSF1R signaling for their survival?
2) If so, then does elimination of microglia improve aspects of disease or recovery from injury? Additionally, what can we learn about the roles of microglia in disease process through their elimination?
We are addressing these questions in mouse models of Alzheimer’s disease and brain injury models.